
![]()
These molecules are also known as coenzyme Q or mitoquinones. They are involved in electron transport in mitochondrial preparations playing an essential role in the oxidation of succinate or NADH via the cytochrome system. They serves not only as a coenzyme but also, in their reduced forms, as antioxidants. They are synthesized de novo in all animal tissues and cannot thus be regarded as vitamins. Ubiquinones are present in all aerobic organisms, plants, animals (the name ubiquinone was proposed with reference to their ubiquitous occurrence) and bacteria, but are absent from Gram-positive eubacteria and the archaebacteria. They were discovered by the Morton’s group (Biochem J 1955, 59, 558) in animal fat but their quinonoid structure was revealed by Crane two years later (Biochim Biophys Acta 1957, 25, 220) in extracts from beef heart mitochondria. The compound had a 2,3-dimethoxy-5-methylbenzoquinone nucleus and a side chain of 10 isoprenoid units and was referred to as coenzyme Q 10 . Later, homologues with 6, 7, 8 and 9 units were isolated from other organisms, bacteria or higher organisms. The main form in man has 10 units but in rat has 9 units. Another system of nomenclature is used: ubiquinone(x) in which x designates the total number of carbon atoms in the side chain, it can be a multiple of 5. Thus, coenzyme Q10 is also named ubiquinone (50). Ubiquinone became the "official" name of the compound in 1975 (IUPAC-IUB commission).
Recent review: "Biochemical, physiological and medical aspects of ubiquinone function" by Ernster L et al. (Biochim Biophys Acta 1995, 1271, 195).
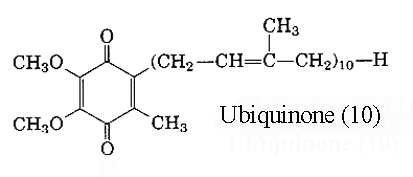
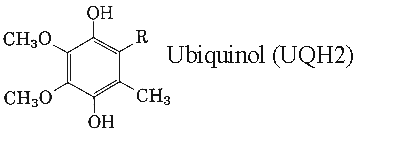
Ubiquinones accept one electron and are transformed into semiquinone radicals (UQH°) or two electrons to give ubiquinol (UQH2)
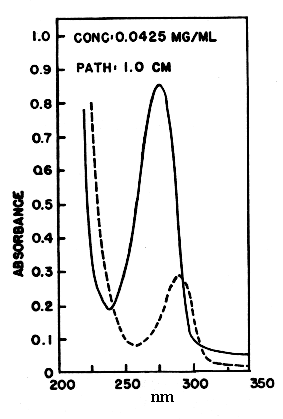
Coenzyme Q is reducible by sodium dithionite or borohydride to its hydroquinone form, and can in turn be reoxidized to the quinone by Ag2O or more slowly by oxygen. The absorption spectra of the two forms are shown below. The quinone form has a strong absorption band at 275 nm which disappears in the reduced form.
 Ubiquinone-9: MW 794
Ubiquinone-9: MW 794
Ubiquinone-10: MW862
Ubiquinones are readily destroyed by heating in alkali , but in the presence of pyrogallol the destruction is minimized.
A new quinone compound with estrogenic properties has been isolated from yam tuber (Dioscorea alata) (Cheng WY et al., J Agric Food Chem 2007, 55, 7350). This product was identified as hydro-Q9 chromene, a derivative of coenzyme Q9.
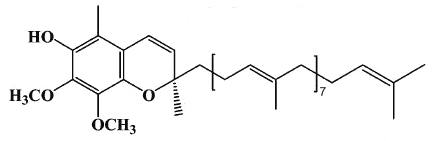
Hydro-Q9 chromene
The presence of that biologically active compound in yam extract likely provides basic evidence for a part of its beneficial effect for menopausal women.
Devenez membre et participez au développement de la Lipidomique au XXIème siècle.
S'inscrire