
VITAMIN A
Vitamin A is a generic term which designates any compound possessing the biological activity of retinol, the animal form of vitamin A. Retinol belongs to a family of chemical compounds known as retinoids.The term retinoids includes naturally occurring forms of vitamin A but also the many synthetic analogs of retinol, even inactive (Sporn MB et al., Fed Proc 1976, 35, 1332). The basic structure of the retinoid molecule consists of a cyclic end group, a polyene side chain with alternating double bonds and a polar end group. This structure is responsible for their color (yellow, orange, or red). Variations in side chains and end groups create the various classes of retinoids.
According to the definition given by IUPA and IUB, retinoids are a class of compounds consisting of four isoprenoid units (diterpene) joined in a head-to-tail manner. All retinoids may be formally derived from a monocyclic parent compound containing five carbon-carbon double bonds and a functional group at the end of the acyclic portion.
As shown by Moore in 1930 (Moore T, Biochem J, 1930, 24, 692) in nutritional experiments, the biosynthetic precursors of retinoids are plant carotenoids (provitamin A) of which b-carotene is most efficient. That precursor is subjected to oxidative cleavage mainly at its center (at the 15,15′ carbon double bond) to yield two molecules of all-trans-retinal as it was proposed by Karrer P et al. (Helv Chim Acta 1930, 13, 1084). Later, it was also demonstrated the possibility of an eccentric cleavage reaction (at the 9′,10′ carbon double bond) and a stepwise process leading to only one mole of vitamin A per mole of carotene consumed (Glover J, Vitam Horm 1960, 18, 371).
The parent retinoid compound, all-trans–retinol, is a terpenoid (diterpene, a 20-carbon primary alcohol, MW: 286). The predominant forms of vitamin A in biological samples are retinol and esters of retinol having long fatty acyl chains. The synthesis of retinyl esters occurs in the intestine (Huang HS et al., J Biol Chem 1965, 240, 2839), liver (Futterman S et al., J Biol Chem 1964, 239, 4077) and in other organs (Berman ER et al., Biochim Biophys Acta 1980, 630, 36). In the majority of mammals, including man, retinyl esters can only be found in traces in blood (Krasinski SD et al., Am J Clin Nutr 1989, 49, 112). As an exception, the majority of vitamin A is transported as esters in the blood of carnivores (except Hyaenidae and Pinnipedia) : 70% in dog, 94% in silver fox, 87% in raccoon dog and 66% in mink (Schweigert FJ et al., Comp Biochem Physiol 1990, 95A, 573).
In animal tissues, retinyl palmitate is the predominant form, oleic and stearic acids being also present. This compound is hydrolyzed into retinol which undergoes reduction into retinal (reversible reaction), and then esterified to form retinyl palmitate. Activation of the retinol pathway, involves hydrolysis and reversible oxidation of retinol to retinal. Ultimately, retinal may be oxidized into retinoic acid.
11-cis-retinal is present in the retina of the eye, and retinoic acids (several forms exist) are active metabolites found in all tissues.
|
|
|
They are all lipid soluble, unstable in the presence of oxygen. Light catalyzes double-bond isomerization of most retinoids, photochemical reactions may lead to polymerization. They must be handle in inert atmosphere, avoiding acid medium and light. Their maximum UV absorbance is at about 325 nm with a molar coefficient of about 50,000. Retinal is covalently bound to rhodopsin, the protein moiety opsin as to melanopsin which is found in photosensitive ganglion cells where its absorbs blue light most strongly.
One international unit (IU) of vitamin A is defined as 0.3 µg of all-trans-retinol. For nutritional works, a better term is retinol equivalent (RE), which is used to convert all sources of vitamin A and carotenoids in the diet into a single unit. 1 µg of retinol equals 1 RE and is assumed to be equivalent to 6µg of b-carotene.
The mean recommended dietary intake is considered to be about 700µg retinol for man. An historical overview on clinical manifestations induced by vitamin A deficiency may be consulted (Sommer A, J Nutr 2008, 138, 1835).
In mammals, 50-80% of total retinol is present in the liver and especially in stellate cells (about 90% of the total) who regulate the vitamin A plasma concentration (about 2 µM). In adipose tissue, retinol is found as esterified compound in adipocyte lipid drops. Retinal is able to inhibit adipocyte differenciation and fat depot, while retinoic acid is able to activate the nuclear receptor PPAR-RXR (Ziouzenkova O et al., Nat Med 2007, 13, 695).
In mammals, 3,4-didehydroretinol (or vitamin A2) is the main metabolite of retinol, present quite exclusively in skin. This form of vitamin A constitutes 20-25 % of the total retinoid content in normal human epidermis..
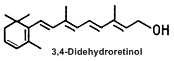
The synthesis of vitamin A2 in epidermis was first demonstrated in 1985 (Torma H et al., J Invest Dermatol 1985, 85, 498), after its discovery in the epidermis in 1980 (Vahlquist A, Experientia 1980, 36, 317). Keratinocytes synthesize vitamin A2 in cell cultures. It appears in both esterified and free forms, similar to retinol. Vitamin A2 is not present in human plasma. Increased abundance of vitamin A2 in the epidermis is correlated with hyperproliferative disorders (eczema, psoriasis, basal cell carcinoma) but the exact relationships of these observations in not yet known. Its biological role in normal and diseased epithelial conditions remains to be established.
Photosensitivity in animals is based on the isomerization of 11-cis-retinal to all-trans-retinal when the complex retinal-rhodopsin is exposed to light, this leads to a sequence of events generating a nerve impulse.
It is now well known that a major hydrophobic constituent of the lipofuscin pigments in retinal pigment epithelial cells is the fluorophore, pyridinium bisretinoid (A2E) (Eldred GE et al., Nature 1993, 361, 724). A2E has been quantified in human donor eyes, and a photoisomer, iso-A2E, has been characterized (Parish CA et al., Proc Natl Acad Sci 1998, 95, 14609). That the accretion of A2E has consequences for the cell, with the adverse effects of A2E being attributable to its amphiphilic structure and its photoreactivity, is consistent with evidence of an association between atrophic age-related macular degeneration and excessive lipofuscin accumulation (review in : Sparrow JR et al., J Lipid Res 2010, 51, 247). A2E is generated by phosphate hydrolysis of the precursor, phosphatidylethanolamine-bisretinoid (A2-PE) (Liu J et al., J Biol Chem 2000, 275, 29354).
Another fluorophore was discovered in the aging retina, the bisretinoid A2-GPE, which has been characterized as sn-glycero-3-phosphoethanolamine (GPE) derivatized by two all-trans-retinal (Yamamoto K et al., Invest Ophthalmol Vis Sci 2011, 52, 9084).
The current understanding of the composition of bisretinoid adducts, the strucure of the various bisretinoids and their biosynthetic pathways have been reviewed (Sparrow JR et al., Prog Retin Eye Res 2012, 31, 121).
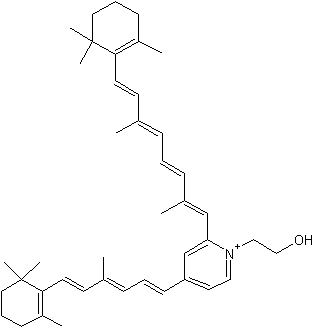
Pyridinium bisretinoid (A2E)
Bisretinoid constituents of the lipofuscin of retinal pigment cells are known to photodegrade into mixtures of aldehyde-bearing fragments and small dicarbonyls (glyoxal) and methylglyoxal. It has been demonstrated that there is a link between the photodegradation of bisretinoid fluorophores and aging changes in underlying Bruch’s membrane (basement membrane of the retinal pigment epithelium) that can confer risk of age-related macular degeneration (Zhou J et al., J Biol Chem 2015, 290, 27215).
Besides these roles, retinoids regulate the growth and differentiation of normal, premalignant, and malignant cells. An overview of the signaling pathway of retinoic acid and retinoid metabolism may be consulted (Theodosiou M et al., Cell Mol Life Sci 2010, 67, 1423). Genomic and nongenomic effects of vitamin A and retinoids have been reviewed (Tanoury ZA et al., J Lipid Res 2013, 54, 1761). A review includes various aspects of retinoid biochemistry in mammals, primarily on retinol and retinyl ester metabolism (O’Byrne SM et al., J Lipid Res 2013, 54, 1731).
In 1987, two groups (Petkovich M et al., Nature 1987, 330, 444-450; Giguere V et al., Nature 1987, 330, 624-9) described a retinoic receptor in the cell nucleus. Since that date, several categories were discovered. The interaction of retinoic acid with its receptors leads to changes in gene expression. A review summarized the roles of various biosynthetic and catabolic enzymes in the regulation of retinoic acid homeostasis (Kedishvili NY, J Lipid Res 2013, 54, 1744).
Devenez membre et participez au développement de la Lipidomique au XXIème siècle.
S'inscrire