
The supercritical fluid extraction (SFE) has been applied only recently to sample preparation on an analytical scale.
This technique resembles Soxhlet extraction except that the solvent used is a supercritical fluid, substance above its critical temperature and pressure. This fluid provides a broad range of useful properties. One main advantage of using SFE is the elimination of organic solvents, thus reducing the problems of their storage and disposal in the lipidologist laboratory. Furthermore, several legislative protocols (such as the EPA Pollution Prevention Act in the USA) have focused on advocating a reduction in the use of organic solvents which could be harmful to the environment.
Besides ecological benefits, one of the most interesting properties of SFE is the high diffusion coefficients of lipids in supercritical fluids, far greater than in conventional liquid solvents. Thus, the extraction rates are enhanced and less degradation of solutes occurs. Several studies have shown that SFE is a replacement method for traditional gravimetric techniques. In addition, carbon dioxide, which is the most adopted supercritical fluid has low cost, is a nonflammable compound and devoid of oxygen, thus protecting lipid samples against any oxidative degradation.
A review of the main theoretical and technical aspects of SFE and SFC (supercritical fluid chromatography) with several interesting links may be found at IsoPro International.
Useful information on SFE may be found on the A.L. Counsulting web site (“The portal of the supercritical extraction technology”).
A review of several applications of SFC for lipid analysis may be consulted (Hartmann A et al., Planta Medica 2015, 23 apr).
The first guiding principle is the optimization of the solubility of lipids in supercritical CO2 and the improvement of the fractionation with respect to a particular lipid species.
Important data on the solubility of vegetal oils and other lipids may be found in the detailed study of Stahl et al. (Dense gases for extraction and refining, Springer Verlag, Heidelberg, 1987).
The graph below shows the dependence of soybean oil triglyceride solubility in supercritical CO2 as a function of temperature and pressure (King JW, Grasa y Aceites 2002, 53, 8).
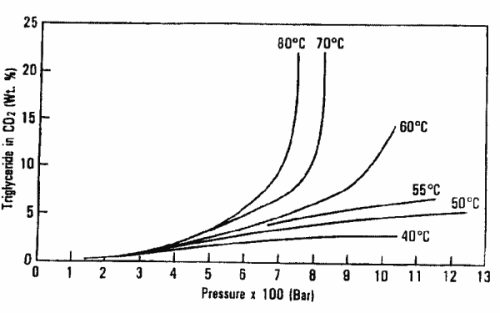
These data have led to perform oil and fat extractions above 600 bar and temperatures from 80 to 100°C.
It should be emphasized that many lipid solutes have similar solubility parameters, making their separation by SFE difficult. An improvement in the separation of complex lipid mixture may be found by the integration of adsorbent compound into the extraction cell with the sample. Several compounds such as alumina, silica, Celite, Florisil or synthetic resins were proposed for the optimization of lipid differential extraction.
The improvement of lipid SFE needs also the study of the kinetics of the lipid removal from the sample matrix. The graph below illustrates the extraction of fat from a low fat matrix containing about 70% water (King JW, Grasa y Aceites 2002, 53, 8).
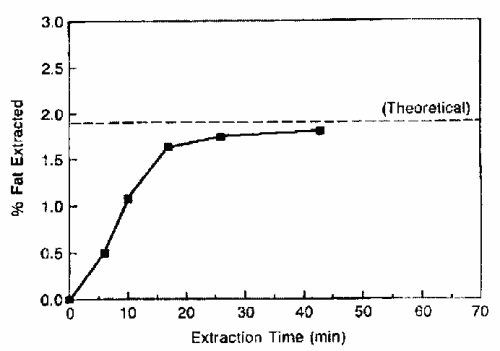
It must be noticed that the fast back-diffusion of analytes in the supercritical fluid reduces the extraction time since the complete extraction step is performed in about 20 min instead of several hours.
A common practice in SFE, which must be mentioned in connection with the physicochemical properties of supercritical fluids, is the use of modifiers (co-solvents). These are compounds that are added to the primary fluid to enhance extraction efficiency. Thus, the addition of 1 to 10% of methanol or ethanol to CO2 expands its extraction range to include more polar lipids. When the extraction was performed with supercritical carbon dioxide and 20% of ethanol, more than 80% of the phospholipids were recovered from salmon roe (Tanaka Y et al., J Oleo Sci 2004, 53, 417).
Those wishing to build their own experimental equipment should read the detailed study of Hawthorne et al. (in Practical supercritical chromatography and extraction, Caude M., Thiebaut D, Eds, Harwood Academic, 1999).
ISCO Inc was one of the first companies to address the off-line SFE market. Extraction cells of 0.5 up to 15 ml are offered. An automatic version permits automated valve operation for extraction of one or two samples and another model permits 24 samples to be automatically and sequentially extracted. ISCO has also offered a extraction system (FastFat HT) specifically made for the rapid determination of fat content in foods and agriculture products (“The Isco FastFat HT provides rapid, accurate % fat determination in a wide range of foods – a critical parameter in quality control, product labeling, and calibrating on-line process control equipment. With FastFat HT, you get reliable results in minutes, and eliminate costly delays in production decisions”). Several application notes concerning SFE of oil and fat from food and agriculture products may be downloaded from the ISCO “Application Highlights” page.
APPLIED SEPARATION offers several extraction units offering great flexibility with respect to sample size and experimental design. Extractor cell sizes can range from several ml to one liter. Off-line and in-line trapping of solutes may be realized.
LECO CORPORATION is offering a total fat analyzer designed with a triple parallel channel system (“LECO’s new TFE2000 Fat/Oil Determinator is specifically designed to determine fat/oil content and eliminate the need for hazardous chemicals used in Soxhlet, Mojonnier/Roese-Gottlieb, acid hydrolysis, and other solvent extraction methods. Using inexpensive compressed CO2 as the solvent, the analyte is extracted from the sample and transferred to the removable ultra efficient solid phase collection traps. Precise calculations are determined by the integrated balance based on sample weight, and the before and after weights of the collection vials”). Application notes may be found on the LECO site.
The use of high purity SFE-grade CO2 is not required but impurity and moisture in industrial grade CO2 can accumulate and may interfere with further analytical operations (gas or liquid chromatography). Thus, an on-line fluid cleanup system may be used to remove trace contaminants.
Extractions can be performed in static, dynamic or recirculating mode. During static extraction, the cell is filled with the supercritical fluid, pressurized and allowed to equilibrate. In the dynalic mode, the fluid is run continuously through the cell, and in the recirculating mode, the same fluid is repeatedly pumped in the cell before being pumped out to the collection vial.
A – Sample matrix
The levels of lipid content and moisture are important for the analytical process. Lipid levels may be from 1 up to 50% (w/w).
B – Sample preparation
Moisture may inhibit contact between extraction fluid and sample. Thus, removal of water by freeze drying is recommended prior to SFE.
It may be also efficient to disperse the sample matrix, to grind the sample to increase the mass transfer of lipids. The choice of drying agent can be made by consulting the study of Bulford (Bulford MD et al., J chromatogr A 1993, 657, 413).
C – Collection of the lipid extract
The collection method for the resultant extract must be optimized to avoid incomplete extraction. The open vials are most frequently used, a sub-ambient cooling being useful for volatile species. It is best to use a collection vial packed with a surface area material, i.e. glass beads, glass wool (Snyder J.M. et al., JAOCS 1994, 71, 261) or containing some volume of a chosen solvent. The supercritical CO2 can be separated from the collected analytes without trouble or disposal problems.
Several reviews were devoted to the application of SFE for the extraction of lipids in various sample types. Those of Clifford A et al. (in Supercritical fluid technology in oil and lipid chemistry, AOCS Press, chap. 19, 1996), Eller FJ et al. (Sem Food Technol 1996, 1, 145) and King JW (Grasas y Aceites 2002, 53, 8) should be consulted for specific applications.
Food and various agricultural products were successfully analyzed for their fat and levels by SFE. Progressively, SFE may be regarded as an alternative to organic solvent extraction methods. The extraction efficiencies of SFE and organic solvent methods (Soxhlet or liquid-liquid extractions) were frequently compared and shown to be in good agreement (Eller FJ et al., J Agric Food Chem 1998, 46, 3657). It must be noticed that gravimetric-based results can be influenced by the sample matrix, the moisture and non-lipid moieties and the extraction solvent.
Total fat determinations were done in meat products with two different extractors and compared with a standard method (Berg H et al., J AOAC Int 2002, 85, 1064). The amount and the composition agreed well with results from the standard procedure. To obtain quantitative recoveries by SFE, 1 ml ethanol was added to 1 g sample in the extraction cells before extraction.
Extracting conditions largely affected the composition of dissolved lipids in freeze-dried animal samples (Tanaka Y et al., J Oleo Sci 2003, 52, 295). Thus, one group of triglycerides was extracted from salmon roe while another group remained in the matrix together with phospholipids and a large part of astaxanthin. It is now well known that the extraction yield and composition of triglycrides depend on the extracting conditions. This was verified for the extraction of oil from seaweed (Cheung PCK et al., J Agric Food Chem 1998, 46, 4228), tomato seeds (Bhupesh CR et al., J Food Sci Technol 1996, 31, 137) or soyabean oil (Snyder JM et al., JAOCS 1984, 61, 1851).
When compared with three other extraction procedures, supercritical carbon dioxide extraction enables an optimal recovery of unsaturated fatty acids (mainly oleic acid, linoleic acid and linolenic acid) in Nitraria tangutorum (Zygophyllaceae) seed lipids (up to 79%) (Suo Y et al., Eur J Lipid Res 2010, 112, 390).
The extraction and separation of phospholipids from tuna fish have been described using various concentrations of methanol in supercritical CO2 (Tanaka Y et al., J Oleo Sci 2005, 54, 569). Good recovery of DHA-rich phospholipids were claimed in a series of industrial processes.
After a supercritical CO2extraction step of neutral lipids, the phospholipids from krill (Euphausia superba) were extracted with a modified existing method using a multi-step procedure using ethanol, hexane and acetone as solvents (Ali-Nehari et al., Korean J Chem 2012, 29, 918).
A detailed description of a reliable procedure adapted for the determination of total fat in milk- and soy-based infant formula powder has been published by LaCroix DE et al. (J AOAC Int 2003, 86, 86).
The extraction of a specific lipid moiety is generally influenced by substances which are co-extracted, all having a high solubility in supercritical CO2.
One technique to overcome this problem is to use a sorbent to retard the analyte of interest, the interfering substances being removed first. Then, a higher CO2 extraction density or a co-solvent is used to removed the analyte of interest from the sorbent. Thus, it was shown that NH2-bonded silica is able to retard sterols.
Analytical SFE has also been used for lipid-derived volatiles such as aroma in various food samples. For the application of SFE to the study of total fat and lipid classes in meats, the studies of Berg H et al. (J Chromatogr A 1997, 785, 345) and Chandrasekar R. (JAOAC Int 2001, 84, 466) should be consulted.
Extraction of pollen lipids by SFE-CO2 was shown to be very efficient for the determination of free fatty acids by HPLC using fluorescence detection (Wang X et al., Eur J Lipid Sci Technol 2009, 111, 155).
Devenez membre et participez au développement de la Lipidomique au XXIème siècle.
S'inscrire