
This group consists of phosphorus-containing sphingolipids (mainly sphingomyelin) but containing a ceramide linked to a phosphate group, itself esterified to a small polar head group (choline, ethanolamine, glycerol). The simplest form, ceramide phosphate, is an important bioactive molecule. The ceramide part is formed by a long-chain fatty acid linked to the amino group (i.e. N-acyl or amide) of a long-chain base. An important review gives an overview of essential elements of sphingolipid structure and analysis, metabolism, functions, and roles in disease (Hannun YA et al., J Lipid Res 2025, 66, 100813).
The sphingosyl phosphatides containing also a glycoside moiety are considered elsewhere. A group of analogues with a C-P bond instead of a C-O-P bond, the phosphonolipids, are present in protozoa and invertebrates.
Ceramide-1-phosphate was first shown to be produced form sphingomyelin by the sphingomyelinase D found in spider venom (Kurpiewski G et al., Biochem Biophys Acta 1981, 678, 467). It was suggested that the spider-induced dermonecrosis near the injection could result from an induced platelet aggregation. Ceramide-1-phosphate was shown to be present for the first time in human leukemia cells (30 pmol/106 cells) and metabolic studies have demonstrated that he was produced by phosphorylation of ceramide by a specific ceramide kinase (Dressler K et al., J Biol Chem 1990, 265, 14917). Later, ceramide-1-phosphate was shown to be a specific and potent inducer of arachidonic acid and prostanoid synthesis in cells (Pettus BJ et al., J Biol Chem 2003, 278, 38206) through the translocation and activation of the cytoplasmic phospholipase A2 (cPLA2) (Pettus BJ et al., J Biol Chem 2004, 279, 11320). Furthermore, it has been suggested that ceramide-1-phosphate and sphingosine-1-phosphate may act in concert to regulate the production of eicosanoids, the important inflammatory mediators (Chalfant CE et al., J Cell Biol 2005, 118, 4605). Studies support the view that a regulated increase in ceramide-1-phosphate during phagocytosis may destabilize the membrane and lead to fusion (Hinkovska-Galcheva V et al., J Biol Chem 2005, 280, 26612).
sphingomyelin has a long-chain base : sphingosine, dihydrosphingosine (in animals) or phytosphingosine (in plants). About two-thirds of the fatty acids found in grey matter sphingomyelin consist of stearic acid while this proportion is formed by lignoceric (24:0) and nervonic (24:1) acids in white matter of the brain. This phospholipid occurs in appreciable quantities in brain (more than 10% of the total phospholipids), but in lesser quantities in other tissues. An exception is the bovine erythrocyte where sphingomyelin forms about 50% of total phospholipids.
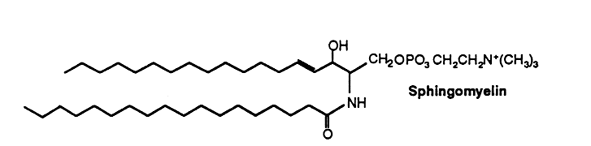
Sphingomyelins containing polyenic very-long chain fatty acids are present in testes and spermatozoa of mammals. The composition of sphingomyelins differs between animal types. Human spermatozoa contained n-6 fatty acids with 2 to 4 double bonds and carbon chain lengths up to 32. In ram and bull testes and spermatozoa, n-3 and n-6 fatty acids with 4, 5 and 6 double bonds with carbon chain lengths up to 34 are found. In rat and boar, the fatty acids are mainly of the n-6 series with 3 to 5 double bonds and carbon chain lengths up to 34. In the latter two animal species, sphingomyelins may contain 2-hydroxylated fatty acids with 4 to 6 double bonds and carbon chain lengths up to 34 (Robinson BS et al., J Biol Chem 1992, 267, 1746). It has been determined that in bull and ram spermatozoa, the very long-chain polyunsaturated fatty acids were the major acyl groups (about 70%) of sphingomyelins and ceramides (Furland NE et al., J Biol Chem 2007, 282, 18151). Experiments on cryptorchid rat testes have shown that these lipids lost their characteristic long-chain and very long-chain fatty acids, notably 22:5n-6 and 28:4n-6, which suggests that these species are linked to the membranes of germ cells (Furland NE et al., Biol Reprod 2007, 77, 181).
It is noticeable that sphingomyelin in the human lens represents almost 70% of all lens phospholipids (2300 nmol per g of tissue), in contrast of the other mammalian species where sphingomyelin is found at a relatively narrow concentration range of 15–29% of total phospholipid (300– 470 nmol per g of tissue) (Deeley JM et al., Biochim Biophys Acta 2008, 1781, 288). Human lenses are dominated by dihydrosphingosine-containing sphyngomyelins, with the species d18:0/16:0 being the most abundant. It has been shown that whale lens membranes have a high sphingomyelin content that may confer resistance to oxidation, allowing these lenses to stay clear relatively longer than many other species (Borchman D et al., J Lipid Res 2017, 58, 2289).
While sphingosine (d18:1) is the most frequent aminoalcohol found in animal sphingomyelins, Ascidians are characterized by a high proportion of sphingadienine (d18:2) as found in plants (Ito M et al., J Oleo Sci 2009, 58, 473).
Sphingomyelins are important phospholipids in plasma membranes of most cells because of their saturated nature which affects the lateral structure of these membranes, and contribute to the regulation of cholesterol distribution within membranes and in cells. Efforts are currently done to correlate the molecular structure of sphingomyelin with functional properties (review in Slotte JP, Prog Lipid Res 2013, 52, 206).
Until recently, sphingomyelin has been thought to be an inert constituent of cell membranes, but current studies suggest that metabolites of sphingomyelin are involved in the signal transduction pathway. The turnover of sphingomyelin involves removal of the polar head, phosphorylcholine, by various sphingomyelinases (phospholipase C-like), generating ceramides or ceramide phosphates.
A sphingolipid analogue of phosphatidylethanolamine, ceramide phosphorylethanolamine, has been reported for the first time in the housefly, Musca domestica (Crone HD et al., Biochem J 1963, 89, 11). The initial finding of this lipid in bacteria was made in Bacteroides ruminicola (Kunsman JE et al., Abst Amer Chem Soc 1966, 152nd meeting, C-255). Shortly later, its correct composition was described and evidence was reported that this sphingolipid represented a significant proportion of the lipids (about half of phospholipids) in another anaerobe, Bacteroides melanogenicus (now Prevotella melaninogenica) (LaBach JP et al., J Lipid Res 1969, 10, 528). Their base appears to have branched or normal saturated carbon chains of 17, 18, or 19 atoms, their fatty acid having a high percentage of 14:0, 17:0, or 18:0 acid. That analogue of phosphatidylethanolamine was reported for the first time in rumen protozoa in 1967 (Dawson RM et al., Biochem J 1967, 105, 837).
This sphingolipid was reported to be also present in snails, marine bivalves (Itasaka O et al., J Biochem 1973, 73, 191), insects (Malgat et al., J Lipid Res 1986, 27, 251) but also in chicken and rat liver (Muehlenberg BH et al., Can J Biochem 1972, 73, 191). It was recently identified in some pathogen fungi (Oomycetes) where its structure was ascertained by mass spectrometry and NMR spectrometry (Moreau RA et al., Lipids 1998, 33, 307). The most abundant molecular species contained a 16-carbon di-OH sphingoid base and erucic acid (22:1 n-9) as the amide-linked fatty acid. Other fatty acids were found: 16:0, 18:0, and 22:0. In another fungi, the long-chain base was determined to be an unusual 19-carbon branched tri-unsaturated sphingoid. It was also extensively studied in lipid extracts from several species of Sphingobacterium (Naka T et al., Biochim Biophys Acta 2003, 1635, 83).
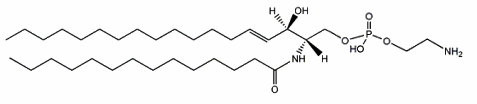
Ceramide phosphorylethanolamine
In mammalian cells, the cellular content of ceramide phosphorylethanolamine is several-hundred-fold lower than sphingomyelin. Nevertheless, its formation through the action of a new form of sphingomyelin synthase is crucial for normal cell functioning, and blocking this enzymatic step results in increased ceramide levels and a defect in the early secretory pathway (Vacaru AM et al., J Cell Biol 2009, 185, 1013).
It was later demonstrated that endosomal delivery of ceramide phosphorylethanolamine with unique acyl chain anchors in spermatocytes is essential for meiotic cytokinesis and spermatid polarity in Drosophilia tissues (Kunduri G et al., PLoS Biol 20(9): e3001599).
Ceramide phosphorylglycerol has been isolated from lipid extracts of an anaerobic bacterium Bacteroides melaninogenicus (LaBach JP et al., J Lipid Res 1969, 10, 528). The long-chain bases appear to have branched (17:0 and 18:0) and normal (18:0) saturated carbon chains while the amide-linked fatty acids have a high percentage of 14:0, 17:0 and 18:0. The presence of this rare lipid, similar to phosphatidylglycerol found extensively in bacteria, has been confirmed later in other Bacteroides species (Kato M et al., Anareobe 1995, 1, 135).
Phosphono analogs of ceramide phosphorylethanolamine have been described particularly in marine invertebrates, including several freshwater and marine bivalves, snails, edible mollusks, jellyfish, shellfish, gastropods of land as well as freshwater and marine origin, and anemones. The first evidence for the occurrence of a phosphonolipid in living materials was that of a ceramide aminoethylphosphonate extracted from a sea anemone, Anthropleura elegantissima (Rouser G et al., JAOCS 1963, 40, 425).
The main phosphono compounds that have been found are ceramide aminoethylphosphonate and its methylated analog ceramide N-methylaminoethylphosphonate. The most abundant fatty acid bound to the amino group is C14 or C16. Sphingoid bases such as d16:1, d18:1, and d19:3 have been detected in squid skin ceramide aminoethylphosphonate (Tomonaga N et al., J Lipid Res 2019, 60, 333). These last authors have studied the absorption of that lipid using a lipid absorption assay on the lymph collected from rats.
).
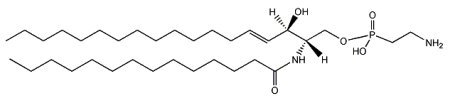
N-(tetradecanoyl)-sphing-4-enine-1-(2-aminoethylphosphonate)
The distribution of these lipids in lower animals may be an important indicator in the phylogenetic field (Hori T et al., Prog Lipid Res 1993, 32, 25). Sphingophosphonolipids have been found in Pelagia noctiluca(Kariotoglou DM et al., Comp Biochem Phys B 2003, 136, 27) and in Aurelia aurita (Kariotoglou DM et al., Lipids 2001, 36, 1255). However, these reports did not provide any detailed information on their structure. The structure of one ceramide aminoethylphosphonate found in the medusa Phyllorhiza punctata has been described (de Souza LM et al., Chem Phys Lipids 2007, 145, 85).
Ceramide methylaminoethylphosphonates from oysters were shown to have in vitro and in vivo anti-angiogenic activities and also to inhibit hormone-dependent and -independent breast cancer cells (Chintalapati M et al., J Agric Food Chem 2009, 57, 5201). These compounds inhibited the viability of hormone-dependent and independent breast cancer cells by inactivating VEGF, EGF, and PI3K, which are some of the main signaling pathways associated with the progression of breast cancer.
Ceramide 2-aminoethylphosphonate present in the internal organs of scallops (Patinopecten yessoensis) has been shown to reduce the serum and liver cholesterol contents in mice (Sugimoto K et al., J Funct Foods 2024, 123, 106569).
A metabolite of sphingomyelin, sphingosylphosphorylcholine, has been shown to be a potentially important lipid mediator in several tissues and immune system. The first description of a remarkably potent mitogenic activity was demonstrated in 1991 (Desai NN et al., Biochem Biophys Res Comm 1991, 481, 361).
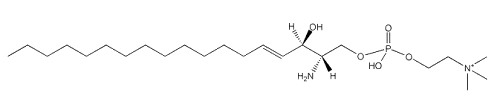
Sphingosylphosphorylcholine
Research has demonstrated that this lyso-phospholipid has no specific receptor but, likely via the sphingosine-1-phosphate receptor, can act as a mitogen and may be also a pro-inflammatory mediator (Nixon GF et al., Prog Lipid Res 2008, 47, 62).
DISPERSIVE LIQUID-LIQUID MICROEXTRACTION
Lire la suiteDevenez membre et participez au développement de la Lipidomique au XXIème siècle.
S'inscrire