
B – HETEROARENES
These hydrocarbons include either oxygen or nitrogen-heterocycle or both (oxygen and nitrogen)
B1 – Cyclic hydrocarbons with oxygen-heterocycle
These cyclic hydrocarbons include heterocycle(s) containing oxygen
Xanthones : xanthones are a class of oxygen-heterocycles containing a heterocycle containing a dibenzo γ-pyrone moiety. Xanthone derivatives (sometimes referred to as xanthonoids) show a wide variety of biological activities depending on the nature and position of substituents.
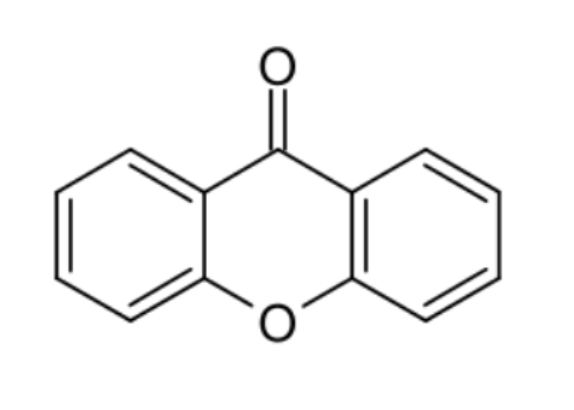 Xanthone
Xanthone
Over 200 natural xanthones have been identified, they are sometimes referred to as xanthonoids. Several are the result of a link between xanthone (or a hydroxylated derivative) and a carbohydrate (these glucosylxanthones are no longer “lipids”), for example mangiferin which has been isolated from the leaves, fruit peels, kernels and bark of Mangifera indica (the mango tree). A Mangifera indica extract (Stadice®) rich in mangiferin was said to enhance cognitive abilities in healthy adults (Jeyakodi S et al., Cureus 16(7): e65751).
Many are found in plants in the families Bonnetiaceae, Clusiaceae, and Podostemaceae, in some species of the genus Iris, and in the pericarp of the mangosteen fruit (Garcinia mangostana). Xanthone is used as an insecticide and it currently as ovicide for some moth eggs and as a larvicide. A great number of bioactive marine xanthone derivatives were reviewed with the description of their structures, biological activities, and marine sources (Soares JX et al., Marine Drugs 2022, 20, 58). Isolation, bioactivities and total synthesis of marine-derived xanthines have been reviewed (Veríssimo ACS et al., Marine Drugs 2022, 20, 347).
Sterigmatocystin is a mycotoxin structurally closely related to the aflatoxins. It contains a xanthone nucleus attached to a bifuran structure (Wikipedia). It has been found in a wide range of food products, with varying levels of contamination observed in rice, wheat, and soybeans. This ubiquitous mycotoxin is synthesized by over 50 fungal genera including Aspergillus, Emericella, Bipolaris, Botryotrichum, Humicola, and Penicillium. It is considered as a potent carcinogen, mutagen, and teratogenIt but it could be a risk to consumers only in special or unusual circumstances (Zhoo Y et al., Food Chem 2025, 19 May, 144814).
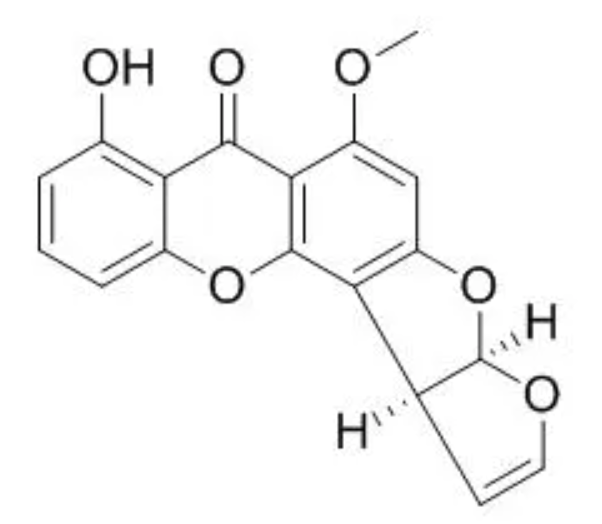 Sterigmatocystin
Sterigmatocystin
Pyran compounds : A saturated pyran ring (a tetrahydropyran), 2,4-dimethyl-5-hexanolide, was shown to be an important and specific trail pheromone in an ant species (Camponotus modoc) (Renyard A et al., J Chem Ecol 2019, 45, 901).
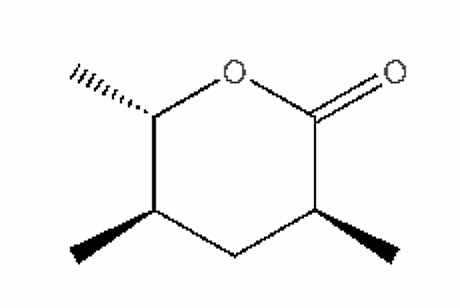
2,4-Dimethyl-5-hexanolide
Benzopyran (Chromene)- This organic and lipidic compound is bicyclic andd results from the fusion of a benzenic nucleus and a pyrane nucleus. In recent years, there have been increasing reports on the application of natural benzopyran compounds in the discovery of new pesticides, especially osthole and coumarin. A systematic and comprehensive review of these active compounds in the discovery of new agricultural chemicals was promoting the discussion and development of benzopyran active compounds (Liu X et al., J Agric Food Chem 2024, 72, 22, 12300).
Coumarins : the simplest compound of this group is coumarin (1,2-benzopyrone). There are several other derivatives by various additions. coumarins belong to the family of lactones (2H-1-benzopyran-2-one, 1,2-benzopyrone or benzo-α-pyrone) and consist of fused benzene and α-pyrone rings. A general classification considering its structure would be simple coumarins, furanocoumarins, pyranocoumarins, dihydrofurano coumarins, phenylcoumarins and bicoumarins. Coumarin belongs to the group of chemicals named chromones or chromanones, They are the derivatives of the true chromone, i.e. 1,4-benzopyrone. That compound, an isomer of coumarin, is a derivative of benzopyrane after substitution by a ketone group on the pyrene cycle.
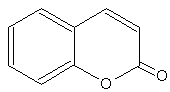
Coumarin
Coumarin is found in many plants, notably in the tonka bean from a tropical tree of the Fabaceae family (Dipteryx odorata) and cinnamon. It is produced also by the Poaceae vanilla grass (Anthoxanthum odoratum) and buffalo grass (Hierochloe odorata), and by a Rubiaceae plant, woodruff (Galium odoratum). All these plants are strongly scented due to the presence of coumarin. Coumarin is also found in many fruit such as strawberries, black currants, apricots, and cherries. As an imitation of vanilla products, it is considered as the center of compositions of the “aromatic fougere” group of scents. The originator of this group is “Fougère Royal” by the house of Houbigant, created in Paris by Paul Parquet in 1882.
Coumarin, used as rodenticide, and extracts from these plants are potential harmful as coumarin is the precursor for several anticoagulants, notably dicoumarol and warfarin.
Coumarins have been used in many research areas, such as cosmetics. It has been banned as a food additive in numerous countries since the mid-20th century. It is still used as a legal flavorant in soaps, rubber products, and the tobacco industry. However, coumarin derivatives have created a major impact in medicinal chemistry, where most of these derivatives have shown interesting pharmacological properties including anticoagulant, anti-inflammatory, antiviral, antioxidant, anticancer and inhibitory of enzymes.
Several coumarins with short- or long-chain hydrophobic groups have been described in roots of Angelica dahurica, a well-known traditional Chinese medicine (Wei W et al., Phytochemistry 2016, 123, 58), some of them having potent anti-inflammatory properties.
Umbelliferone is also known as 7-hydroxycoumarin and is a natural hydroxylated derivative of coumarin. It occurs in many familiar plants from the Apiaceae (Umbelliferae) family such as carrot, coriander and garden angelica, as well as in other plants: the mouse-ear hawkweed (Hieracium pilosella or Pilosella officinarum, Asteraceae) or the bigleaf hydrangea (Hydrangea macrophylla, Hydrangeaceae). It absorbs ultraviolet light strongly at several wavelengths. There are several indications that it is antimutagenic. It has antioxidant properties and absorbs ultraviolet light strongly but despite possible harmful mutagenic properties, is used in sunscreens.and an optical brightener for textiles.
herniarin (7-methoxycoumarin) occurs in the leaves of tha Asteraceae water hemp (Eupatorium ayapana) and in several Herniaria (Caryophyllaceae).
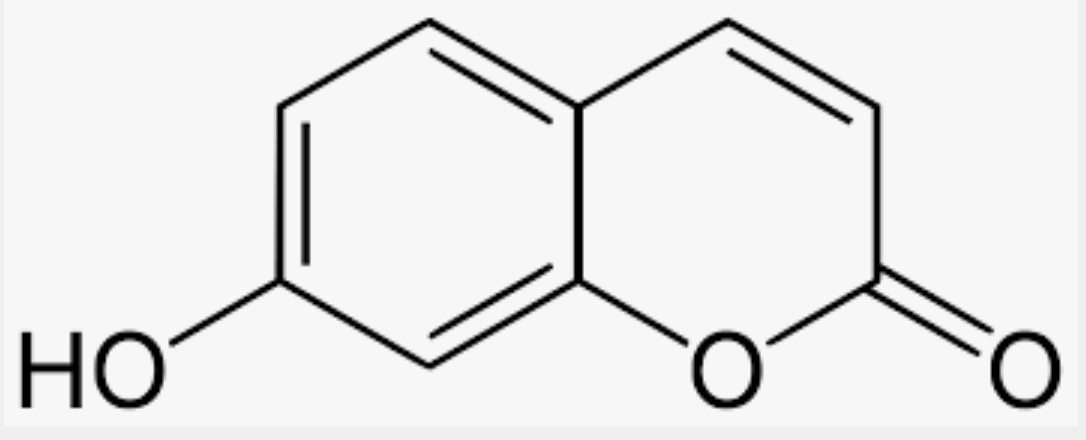 Umbelliferone
Umbelliferone
These compounds are found throughout the plant kingdom, where they give rise to numerous complex molecules. They may provide protection from ultraviolet light, against herbivores and pathogens, and mediation between plant and pollinators.
5-Methylcoumarins isolated and characterised from Clutia lanceolata, a medicinal plant native to sub-Saharan Africa and the Arabian Peninsula, strongly stimulated glucose-triggered release of insulin from β-cells (Ahmed S et al., Phytochemistry 2020, 170, 112213).
It is now well known that the secretion of coumarins is a crucial mechanism for iron uptake in nongrass species when iron availability is low, particularly in alkaline soils. Studies have identified the main coumarins involved in iron uptake and the main biochemical steps and regulatory processes controlling their biosynthesis (Robe K et al., Trends Plant Sci 2021, 26, 248)-.These findings may provide novel targets for improving plant growth and health together with iron content in the edible part of plants and thus human diet.
Several studies have reported a wide range of fusarochromanone derivatives from both terrestrial and marine fungus Fsusarium equiseti strains. The structure of fusarochromanone is unique due to the presence of two geminal methyl groups at C-2 and the alternating β-keto-amine groups. It exhibits potent antiangiogenic and antitumor activity. It has been also demonstrated that fusarochromanone exhibits significant in vitro growth inhibitory effects against glioblastomas and melanomas through induction of apoptosis and different pathways (Mahdavian E et al., BMC Res Notes 2014, 7, 601). New fusarochromanone derivatives with various biological activities are currently discovered (Pham GN et al., Mar Drugs 2024, 22(10), 444).
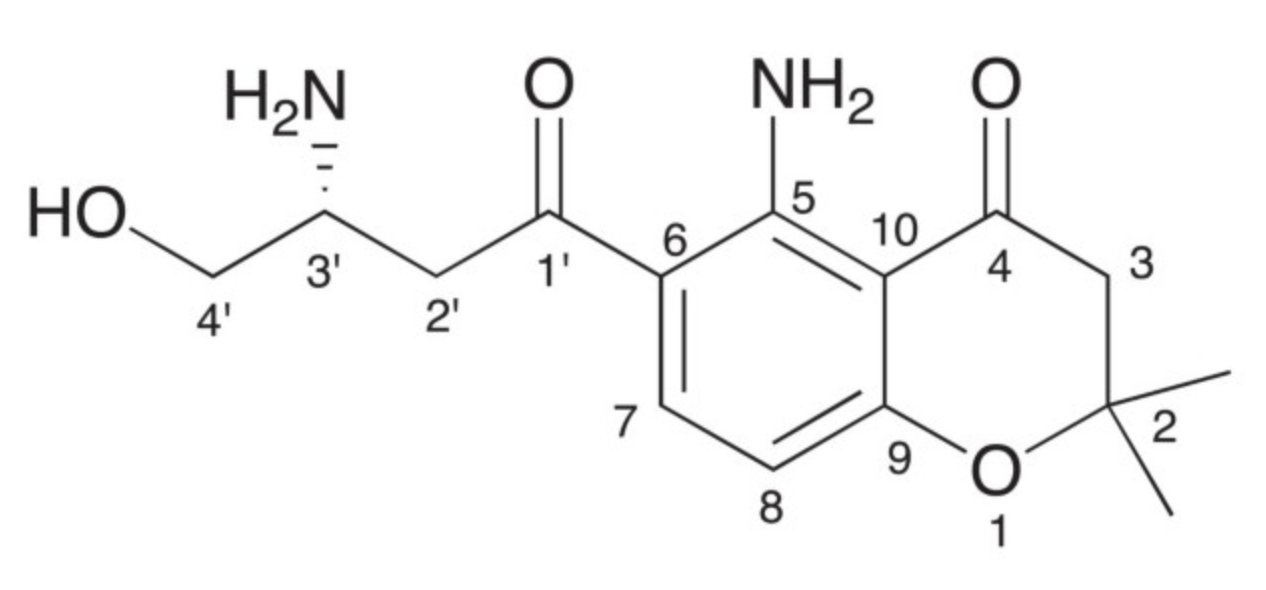 Fusarochromanone
Fusarochromanone
Some coumarin derivatives (phenylpropanoids) are present in various plants. Among them, umbelliferone, aesculetin, herniarin, psoralen and neoflavones. Scopoletin (6-methoxy-7-hydroxycoumarin) is a phytoalexin produced by several Solanaceae, such as tobacco (Nicotania tabacum), which protects plants against virus, bacteria or fungi (Oirdi ME et al., Environ Microbiol 2010, 12, 239).
The biological properties of coumarin derivatives have been studied in a variety of fields. For marine-derived coumarins, their cytotoxic properties are by far the most studied. Coumarins from oceans have been mostly found in coastal plants, bacteria, mollusks, invertebrates and sponges (Murray RD, Prog Chem Org Nat Prod. 2002, 1-619).
A review has showcased the pharmacological activities and relevant synthetic routes for specific families of derivatives and their analogues (Fernández-Peña L et al., Mar Drugs 2023, 21(1), 37). Novel methodologies has been covered to illustrate original synthetic opportunities.
FLAVONOIDS
Many flavonoids (flavones, flavonoid) and isoflavones have a chromone core substituted by a phenyl group in position 2 or 3. Flavones (from Latin flavus “yellow”) are a class of flavonoids based on the backbone of 2-phenyl-1-benzopyran-4-one. Flavones are common in foods, mainly from spices, and some yellow or orange fruits and vegetables. Common flavones include apigenin, naringinin, luteolin, tangeritin, and chrysin (Wikipedia). Flavonoids are the most important plant pigments for flower coloration, producing yellow or red/blue pigmentation in petals. In higher plants, they are also involved in UV filtration, symbiotic nitrogen fixation, and floral pigmentation (Wikipedia). In plants, isoflavones are found mostly (>80%) in the form of glycoconjugates and the corresponding acetyl and malonyl derivatives]. Glycosides are not readily absorbed in the gut and have only low-level estrogenic activity. Isoflavonesare are physiologically functional when these glycosides are hydrolyzed into their corresponding isoflavone-aglycones.
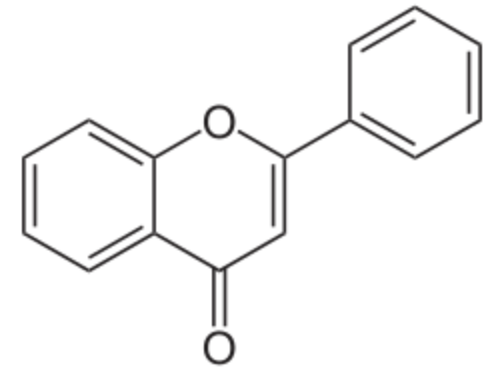 Flavone
Flavone
(2-phenyl-1-benzopyran-4-one)
Luteolin was first extracted from Reseda luteola (Resedaceae) and used as a yellow dye since the first millennium B.C. It was isolated and named, in 1829 by the French chemist Michel Eugène Chevreul. It is present in various fruits, vegetables and natural medicinal materials, including celery, broccoli, artichoke, green pepper, parsley, thyme, dandelion, perilla, chamomile tea, carrots, olive oil, peppermint, rosemary, oranges, and oregano (Wikipedia). Luteolin is known for its anti-inflammatory, antioxidant, and anticancer properties (Sung et al., J Med Food 2015, 18, 557). Lluteolin has also demonstrated promising antidiabetic and antiobesity properties regulating glycolipid metabolism, improving insulin sensitivity, and reducing blood glucose levels (Pradhan G et al., Curr Diabetes Rev, 2025), 21, 10.2174).:A critical review of luteolin’s effects on glycolipid metabolism and the underlying molecular mechanisms may be found (Sojan A et al., J Functional Foods 2025, 132, 106972).
 Luteolin
Luteolin
Cynaroside (also known as luteoloside) is a 7-O-glucoside of luteolin, it has been demonstrated to alleviate oxidative stress and cellular apoptosis across different cell lines and to inhibit cell migration and invasion in human colorectal cancer (Zhong J et al., Food Biosci January 2025, 105915).
Naringenin is a flavone commonly found in citrus fruits, especially in grapefruit. High consumption of dietary naringenin is generally regarded as safe, but consuming grapefruit excessively may impair the action of anticoagulants and increase the toxicity of various prescription drugs. Naringenin may evoke CYP3A4 suppression in the liver and intestines, possibly resulting in adverse interactions with common medications (Wikipedia). It may be found both in the aglycol form, naringenin, or in its glycosidic form, naringin. Due to their capacity to fight apoptosis, inflammation, and oxidative stress, naringin and naringenin are considered valuable therapeutic agents for diabetes mellitus (Fotouhi S et al., J Function Foods 2025, 124, 106643). Naringin has demonstrated a strong potential as a dietary adjunct for cardiovascular protection, especially in the context of ischemic injury and vascular dysfunction (Adams JA et al., Nutrients 2025, 17, 2658).
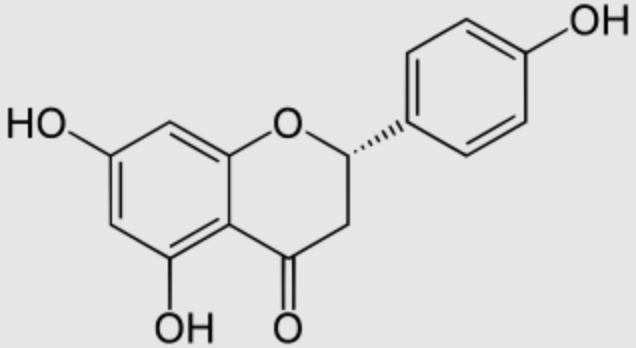 Naringenin
Naringenin
Genistein is a plant-derived, aglycone isoflavone. It has the highest content of all isoflavones in soybeans and soy products. As a type of phytoestrogen, genistein has estrogenic activity in vitro; consequently, its long-term intake by consuming soy products may affect reproductive organs, such as the uterus and breast (Wikipedia). Genistein therapy in rodent models of Altzeimer’s disease has indicated it reduces Aβ deposition and improves memory in preclinical trials (Lei H et al., J Agric Food Chem 2024, 72, 13500).
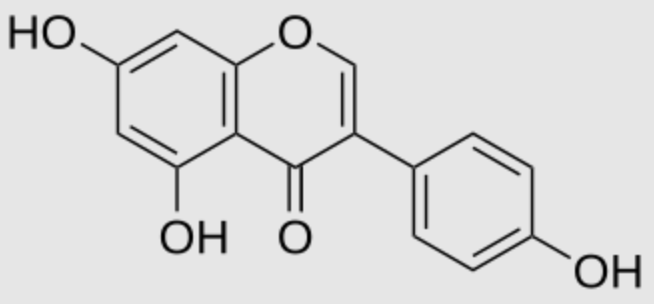 Genistein
Genistein
Equol is an agonist of estrogen receptor α (ERα) and ERβ and an active metabolite of the isoflavone daidzein. It is formed from daidzein by gut microbiota. Equol induces ERα-and ERβ-mediated gene transcription in HEC-1 cells expressing the human receptors, so human exposure to equol could have significant biological effects (Muthyala RS et al., Bioorg Med Chem 2004, 12, 1559). Analogous compounds of equol (5-hydroxy-equol, dehydroequol, 5-hydroxy-dehydroequol) are also bioactive isoflavones formed by microbial metabolism (review in: Mayo B et al., Nutrients 2019, 11, 2231).
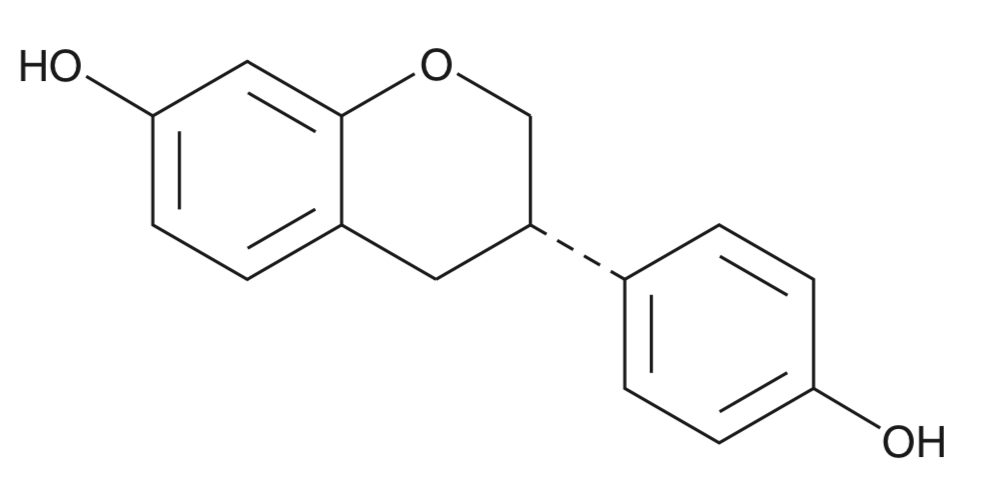 Equol
Equol
Tangeretin is a five times methoxylated flavone that is found in tangerine (Citrus tangerina) and other citrus peels. Tangeretin is commercially available as a dietary supplement. Tangeretin was also shown to have beneficial applications in other pharmaceutical, nutraceutical, and cosmetic processes. It was demonstrated to have diverse biological activities, including antioxidant, anti-inflammatory, anti-tumor, hepatoprotective, and neuroprotective effects. A study has emphasized the potentiel use of that natural chemical as an anxiolytic in mice (Husain Z et al., Food Biosci 15 November 2024, 105469). It exert anxiolytic effects in a dose-dependent manner in open-filed, hole-cross, swing, and dark-light studies in mice by elevating locomotion.
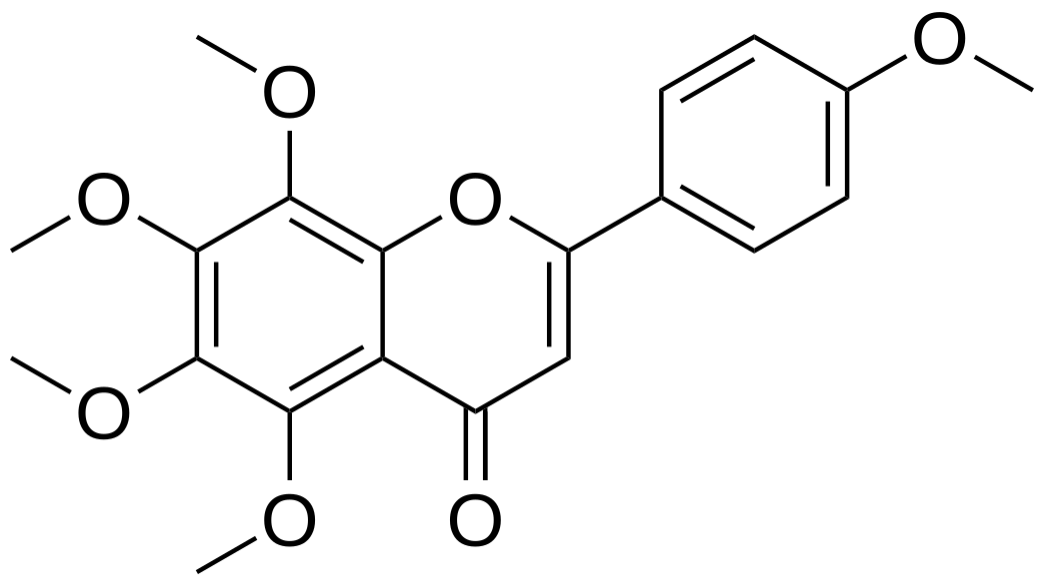 Tangeritin
Tangeritin
Nobiletin is a hexamethoxyflavone isolated from citrus peels. Its bioactive (fungicidal) capacities were demonstrated in citrus fruits, later in humans where it prevents and improves certain degenerative pathologies (Wikipedia). Several observations suggest that nobiletin has the potential to become a novel drug for the treatment and prevention of neurodegenerative diseases such as Alzheimer’s disease and Parkinson’s disease (Nakajima A et al., Int J Mol Sci 2019, 20, 3380).
Nobiletin has also fascinating anti-cancer properties. It has been shown to induce the generation of endogenous ROS by cancer cells, leading to damage to critical macromolecules and finally cell death (review in: Huang J et al., Apoptosis 2022, 27, 297).
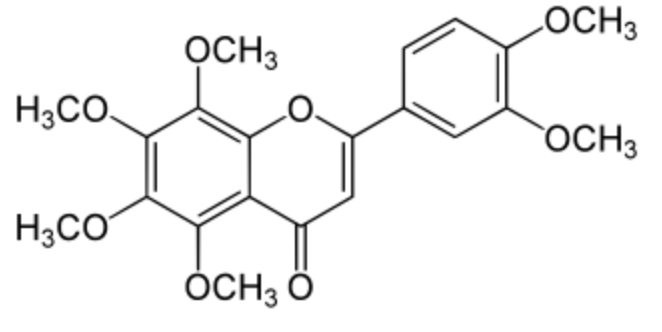 Nobiletin
Nobiletin
Hydrangenol is a flavonoid present in Hydrangea macrophylla (Hydrangeaceae), in free form or as 8-O-glucoside. It has been used to treat allergic reactions. Several studies have reported that it has also anti-inflammatory, anti-diabetic, and anti-malarial activities. A study reported on the mode of action of hydrangenol as an inhibitor of bladder cancer (Shin SS et al., EXCLI Journal 2018;17:531). It was also suggested that hydrangenol can prevent cutaneous wrinkle formation by reducing matrix metalloproteinase and inflammatory cytokine levels and increasing the expression of moisturizing factors and antioxidant genes (Myung DB et al., Nutrients 2019, 11: 2354).
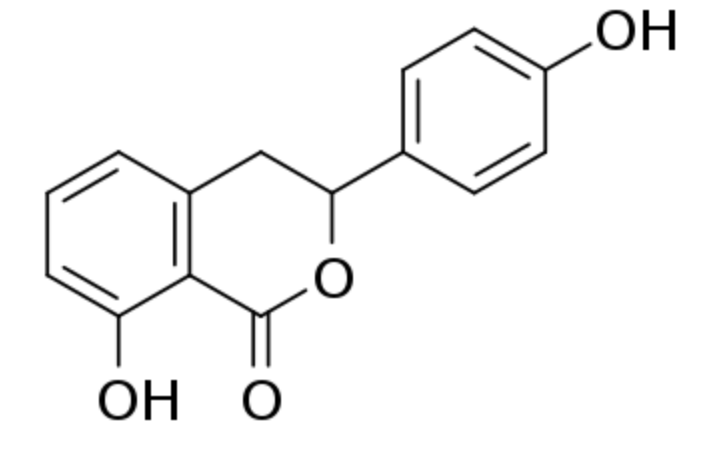
Hydrangenol
Chrysin, also called 5,7-dihydroxyflavone, is found in honey, propolis, and various plants, such as the blue passion flower (Passiflora caerulea) (Wikipedia). Chrysin is used as an ingredient in dietary supplements. Study have demonstrated that it attenuates brain aging induced by D-galactose by enhancing scavenging enzyme activities and reducing oxidative stress, neuronal apoptosis, and the impaired hippocampal neurogenesis (Prajit R et al., Biogerontology 2024 Sep 19). Ffindings underscore an important role of chrysin in inhibiting the proliferation of hepatic cancer cells and shed a light on the strategy for development the functional effects of foods (He K et al., Food Biosci 2024, 105148). The interest in anti-cancer properties of various flavonoids, including chrysin, has been reviewed (Wong SC et al., Nutrients 2023, 15, 797).
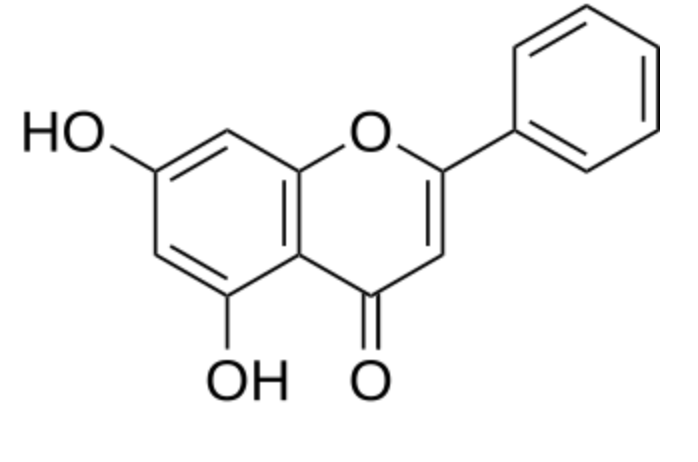 Chrysin
Chrysin
Kaempferol (3,4′,5,7-tetrahydroxyflavone) is a natural flavonol, a type of flavonoid, found in a variety of plants and plant-derived foods including kale, beans, tea, spinach, and broccoli (Wikipedia). It has attracted much attention due to its anticancer, anti-inflammatory, antioxidant, antitussive and other efficacy (Wang Q et al., Biomed Pharmacother 2023, 159,114226). Kaempferol was shown to inhibit oxidative stress and reduce macrophage pyroptosis by activating the NRF2 signaling pathway (Wang Y et al., 06 Jun 2025 PLOS ONE).
It has been observed that the kaempferol derived from Artemisia absinthium has the highest binding affinity towards the insect Helicoverpa armigera acetylcholinesterase. It has been suggested that concluded plant extracts containing that compound possess significant insecticidal properties and could be developed as botanical insecticides (Yaseen M et al., PLoS One 2025, 20, e0325959).
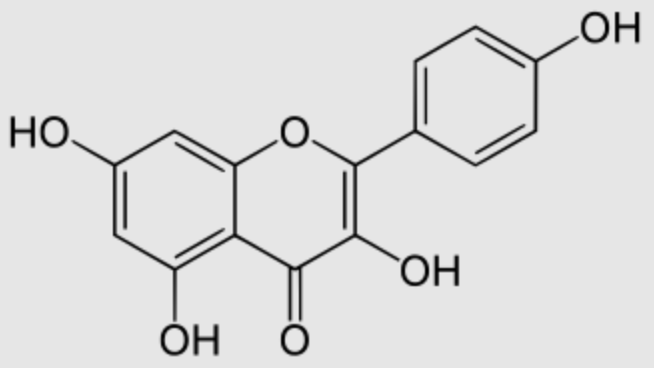 Kaempferol
Kaempferol
Auraptene is a natural bioactive monoterpene coumarin ether. It was first isolated from members of the genus Citrus. It is the geranyloxycoumarine the most abundant in nature (Fiorito S et al., Phytochem Rev 2022, 21, 317). In 1930, Nihon Kagakkai discovered its presence in sweet orange peel (Citrus sinensis). Auraptene has shown some effect as a chemopreventative agent against cancers of liver, skin, tongue, esophagus, and colon in rodent models. Studies have clearly indicated that it exerts significant effects at the central nervous system level, thus having a great potential as a neuroprotective agent.
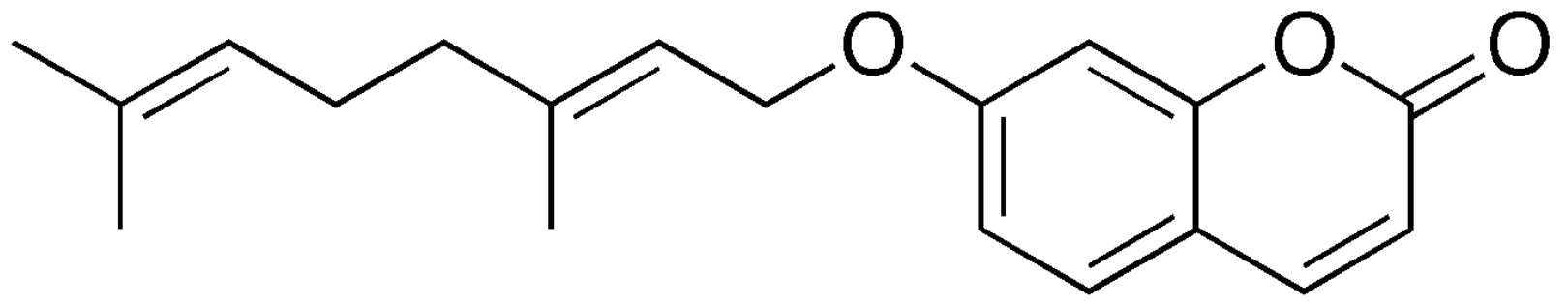 Auraptene
Auraptene
Umbelliprenin (7-farnesyloxycoumarin) has attracted the attention of several research groups due to its anti-inflammatory, immunomodulatory, and anticancer activities (Fiorito S et al., Phytochem Rev 2022, 21, 317).
 Umbelliprenin
Umbelliprenin
This oxyprenylated coumarin is commonly found in different plant species such as Ferula, Peucedanum, Seseli, Magydaris, and Ammi but also in some edible fruits and vegetables such as celery (Apium graveolens L.), wild celery (Angelica archangelica L.), and Citrus lemon. A detailed investigation on its chemical stability and its catabolism has been released (Genovese S et al., J Nat Prod 2017, 80, 2424).
Osthole (or osthol) [7-methoxy-8-(3-methylpent-2-enyl)coumarin] is a coumarin derivative found in Cnidium monnieri, a plant used in traditional Chinese medicine to treat skin affections, and in Angelica archangelica and Angelica pubescens.. Osthole was shown to exhibit several biological functions, including antiosteoporotic, antiallergic, anti-inflammatory, and antitumor functions. Recently, it was found that osthole might be a potent antidiabetic agent (Lee W.H. et al., J Agric Food Chem 2011, 59, 12874).
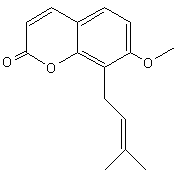
Osthole
Citrinin is a mycotoxin (a benzopyran-7-carboxylic acid derivative) which is often found in food (stored grains, sometimes fruits and other plant products). It is a secondary metabolite produced by fungi that contaminates long-stored food causing various toxic effects, like nephrotoxic, hepatotoxic and cytotoxic effects. It was first isolated from Penicillium citrinum (Wikipedia).
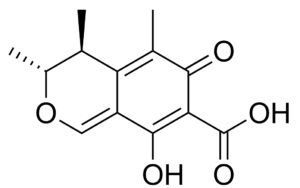
Citrinin
Citrinin was shown to have a broad antibacterial activity. It has been demonstrated that Pseudomonas aeruginosa, an opportunistic pathogen, regulates its virulence factors and biofilms through a quorum sensing system and that citrinin produced by a Penicillium species was able to inhibit the production of virulence factors, biofilm and motility by Pseudomonas (Hongrui Ji et al., Mar Drugs 2023, 21(5), 29).
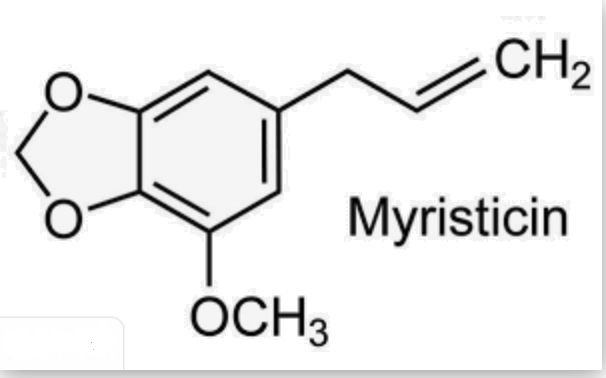
– Myristicin is a phenylpropenoid compound present in small amounts in the essential oil of nutmeg and in several members of the carrot family. It is psychoactive, and acts as an anticholinergic agent. It is a precursor for the illicit synthesis of the psychedelic and empathogenic drug MMDA. Raw nutmeg contains 0.2-1.3% myristicin.
Piperine has a core structure similar to that of myristicin but with a piperidine nucleus (heterocycllic amine). It is the most active and abundant plant alkaloid in the fruit compounds responsible for the pungency of black pepper. Its amount varies from 5–10% in commercial white and black peppers. Under ultraviolet light, piperine is changed into isomers which are tasteless. Piperine was discovered in 1819 by the Danish chemist and physicist Hans Christian Ørsted, who isolated it from the fruits of Piper nigrum (Ørsted will remain famous in discovering that electric currents create magnetic fields, which was the first connection found between electricity and magnetism). The fatty acyl chains within the piperine molecule stimulate sensory nerves, activating capsaicin receptors that initiate nerve signaling, ultimately leading to the perception of pungency (review in : Zou R et al., Food Chem 2024, 456, 139980). Piperine is largely used for its effect on enhancing absorption and bioavailability of other compounds. It has been demonstrated that piperine has protective effect on indomethacin-Induced Intestinal damage. Authors concluded that cotreatment with piperine has great potential in mitigating the side effects caused by nonsteroidal antiinflammatory drugs (NSAIDs) (Gervasoni KN et al., Mol Nutr 2025, 69, e70097).
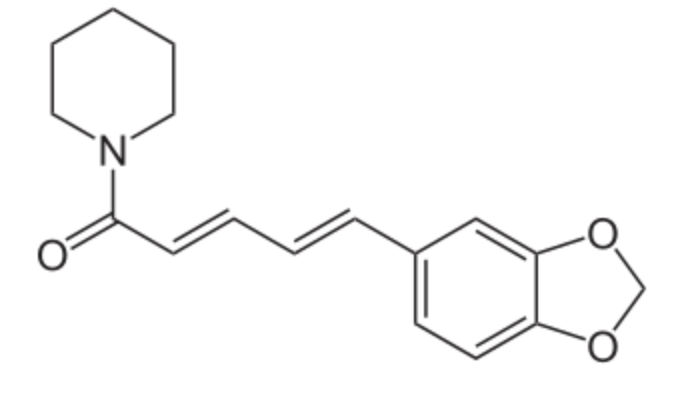
Piperine
– Psoralen (or psoralene) is a furanocoumarin. It is a derivative from umbelliferone by addition of a furan ring. Psoralen has been described in the seeds of the Fabaceae Psoralea corylifolia, but is present in many plants such as several Rutaceae (Ruta, Citrus), Moraceae, Leguminoseae Psoralea, Coronilla) and Apiaceae. Psoralen-rich plants are used in Chinese and Indian medicines and psoralene, due to its UV absorption properties, is used in treatment of psoriasis, eczema, vitiligo and in some cutaneous lymphoma. Psoralen is used in tanning accelerators, but it should be kept in mind that it increases the skin’s sensitivity to light.
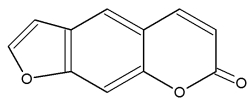
Psoralene
Bergapten is a methoxylated derivative of psoralen (on carbon 5). It is found in bergamot essential oil and in other citrus essential oils. 5-MethoxypsoralenIn was isolated in 1834 by Kalbrunner. It was the first furanocoumarin to be isolated and identified. Contact with plant parts containing bergapten (and other linear furanocoumarins) followed by exposure to ultraviolet light may lead to phytophotodermatitis. According to the International Agency for Research on Cancer, bergapten is probably carcinogenic to humans. Bergapten serves to have the skin absorb more light, and pigmentary diseases like vitiligo (leukodermia) and psoriasis in conjunction with sun exposure or solar radiation
Many furanocoumarins are toxic and are produced by plants as a defense mechanism against various types of predators and in human, some of them (bergamottin) are responsible for the “grapefruit juice effect”, in which they affect drug metabolism. Bergamottin is present in the juice but is more concentrated in the essential oil of bergamot. It is also found in both the skin and flesh of grapefruit and has been reported in flavedo (yellow skin) at concentrations as high as 666 μg/g.
It is a linear furanocoumarins with a side chain derived from geraniol on the carbon 5 of the central ring. Bergamottin and dihydroxylated derivatives have been found to inhibit the human intestinal cytochrome P450 3A4 isozyme (CYP3A4) involved in the metabolism of some prescribed medications (Edwards DJ et al., Drug Metab Dispos 1996, 24, 1287). Bergamottin exhibits several characteristics that are beneficial to human health, such as anti-inflammation (Shen G et al., Front Pharmacol 2024, 15, 1389786), antioxidant, and anti-cancer effects (Santos CM et al., Fitoterapia 2023, 168, 105489). A review is focused on anti-cancer, ant-inflammatory, anti-obesity potential of bergamottin through literature review and in silico studies (Farzeen I et al., Phytochem Rev 2024, 24, 3041).
They are also bactericide, fungicide, antiviral and insecticide and in plant they have an important role as inhibitor of germination.
 Bergamottin
Bergamottin
Pyranonaphthoquinones : they are a large group of over one hundred natural products primarily isolated from bacteria and fungi, many of which have been found to exhibit antibacterial, antifungal and anticancer properties. The basic structure of the pyranonaphthoquinones is the naphtho[2,3-c]pyran-5,10-dione ring system (see below).
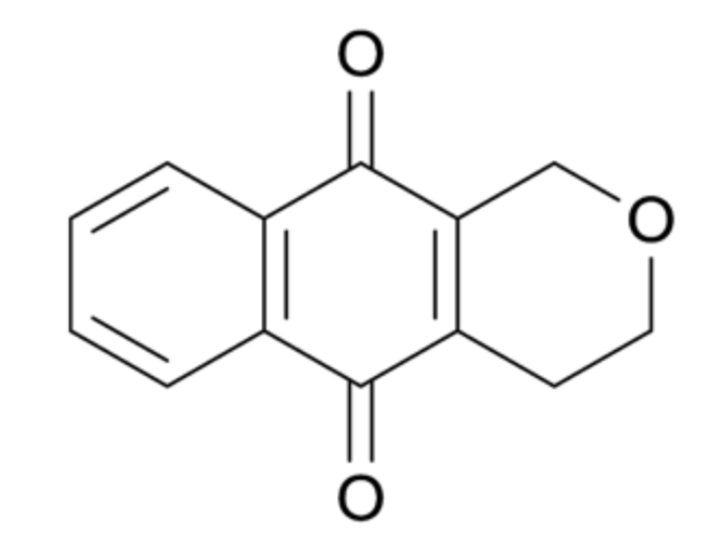
Pyranonaphthoquinone natural products vary in the substitution of the pyran and aromatic rings, and can possess additional fused ring systems. A review discussed the isolation, biological activity and synthesis of pyranonaphthoquinone natural products (Naysmith BJ et al., Nat Prod Rep 2017, 34, 25), Some derivatives, named ventiloquinones, have been isolated from Ventilago harmandiana (Rhamnacea), a plant used in traditional medicine in Thailand, and have shown potent anti-inflammatory activity (Panthong K et al., Phytochemistry 2020, 169, 112182).
Butenolides are modified hydrocarbons with a hetocyclic nucleus of the furan type. The simplest one is 2-furanone.
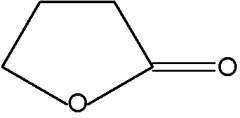
2-Furanone
That compound is characteristic of aroma of Apiacaea (genus Angelica). It can also inhibit the quorum sensing in bacteria. Several analogues or derivatives of 2-furanone are present in fruit aroma : 4-methoxy-2,5-dimethyl-3-furanone in pineapple and 4-hydroxy-2,5-dimethyl-3-furanone (furaneol) in strawberry and tomato. Furaneol is present in roasted peanut and tobacco smoke and 3,5,5’-trimethyl-2-furanone appears in roasted in hazel-nut. Some other derivatives are active as sexual pheromones in insects.
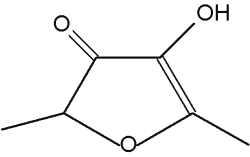
Furaneol
Several derivatives of 2-furanone with a polyacetylenic side chain have been isolated from Asteraceae (Vernonia scorpioides) and displayed antiherpeetic activities (Pollo LAE et al., Phytochemistry 2013, 95, 375).
A review of the naturally occuring furanones may be consulted (Slaughter JC et al., Biol Rev 1999, 74, 259).
Several furan and furanone derivatives have been isolated from Persea species (Bhuyan DJ et al, Antioxidants 2019, 8, 426). They have notable insecticidal, cytotoxic, and antifungal activities, the saturation of the furan ring being detrimental for the insecticidal activity.
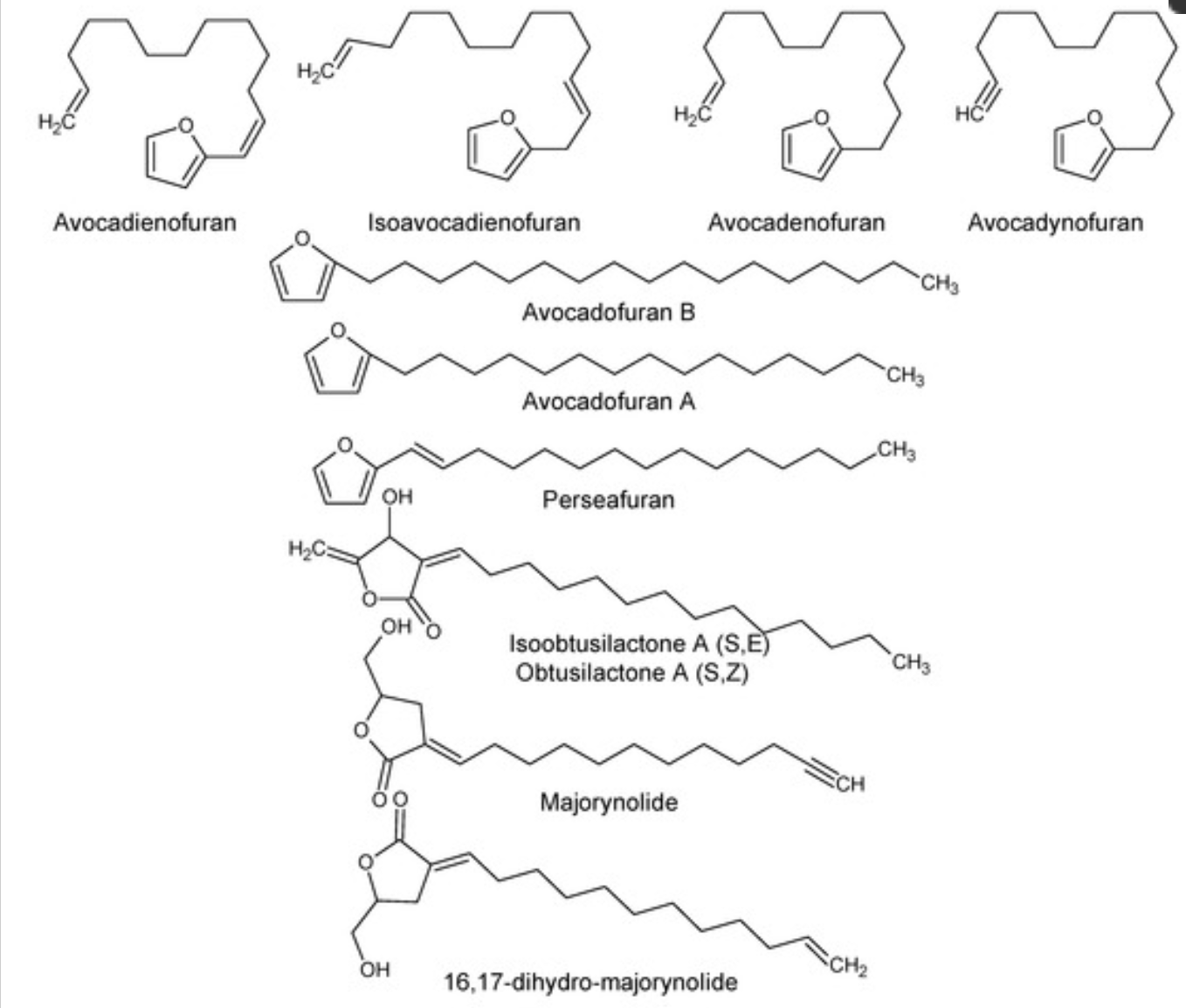
Furan and furanone derivatives isolated from avocado
Acetogenins are polyketide products mainly found in plants of the family Annonaceae. They have a linear 32- or 34-carbon chain containing oxygenated functional groups and terminated with a lactone or a butenolide group and one to three tetrahydrofuran rings along the hydrocarbon chain (Wikipedia). Some of them extracted from avocado were shown to have interesting nemetocidal activities (Hunashal Y et al., Food Chem 2025, 491, 145215).
One of the best known is Annonacin which is present in fruits of plants from the family Annonaceaea. It has toxic effects on the nervous system which could be related to atypical Parkinsonism (Wikipedia).
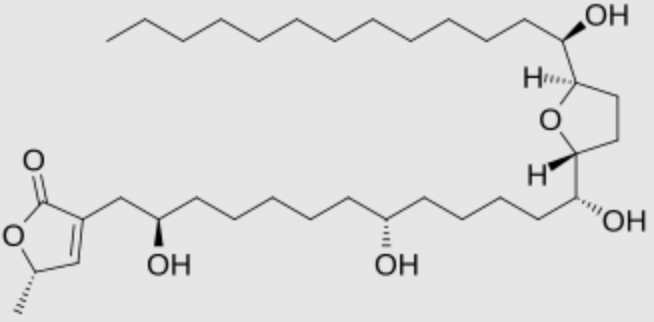 Annonacin
Annonacin
Karrikins are a butenolide family of plant growth regulators found in smoke derived from burning plant material (mainly cellulose) (Flematti GR et al., Science 2004, 305, 977). These compounds are derived from 2-furanone by condensation with a pyrane group. They act as a key germination trigger for many species from fire-prone plants. It was later shown that karrikins act by a mechanism requiring gibberellic acid synthesis and light (Plant Physiol 2009, 149, 863). The most active karrikin is 3-methyl-2H-furo[2,3-c]pyran-2-one (see below). Due to the fact that it has an effect at extremely low concentrations (as low as 1 nM), it has potential as an important agronomic and horticultural chemical. A parent compound (3,4,5-trimethylfuran-2-one) has been also isolated from plant-derived smoke, but it has inversely a germination inhibiting property (Light ME et al., J Nat Prod 2010, 73, 267). The interaction of these compounds may have important ecological implications.
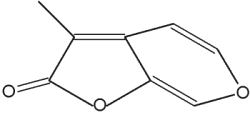
Karrikin
Brominated furanones are produced by Delisea pulchra, a marine alga endemic to the south-eastern coast of Australia. Some of which have strong inhibitory activity against fouling organisms and herbivores (de Nys R et al., Tetrahedron 1993, 49, 11213). Additionally, furanones have specific effects on colonization phenotypes of marine bacteria at concentrations found on the surface of the alga (Maximilien R et al., Aquat Microb Ecol 1998, 15, 233). Manefield M et al. have shown that these compounds inhibit acylated homoserine lactone-mediated gene expression in Escherichia coli (Manefield M et al, Microbiology 1999, 145, 283).
Extracts from the fruit of Choerospondias axillaris were shown to contain alkylated derivatives from benzofuran which displaid potent antiproliferative and cytotoxic activities against Ewing sarcoma and medulloblastoma cells, the MCT1 transporter being their target (Kil YS et al., J Nat Prod 2020, 83, 584).
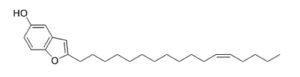 One of the most potent compounds among alkylated benzofurans
One of the most potent compounds among alkylated benzofurans
Ligustilide, a benzofuran (or phthalide) compound, is an active ingredient of umbelliferous plants such as Angelica sinensis and Ligusticum chuanxiong and is considered as the most effective biologically active ingredient in these plants widely used in traditional Chinese herbal medicine (Yang F et al., Scientific Reports 2019, 9, 6991). Ligustilide has been found to have multiple pharmacological activities, such as anti-atherosclerosis, neuroprotection, anticancer, anti-inflammatory and analgesic. Ligustilidehas been also able to significantly improved neurological function in promoting angiogenesis in vitro and in vivo (Changhong Ren et al., Neurol Res 2020 Jun 25;1-10).
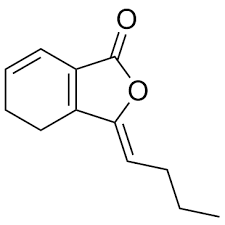
Ligustilide
Dibenzofuran is an aromatic compound that has two benzene rings fused to a central furan ring. All the numbered carbon atoms have a hydrogen atom bonded to each of them. It is a volatile white solid that is soluble in nonpolar organic solvents. It is obtained from coal tar, where it exists as a 1% component.
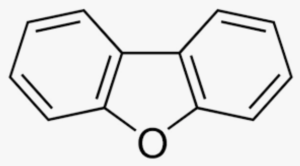
Dibenzofuran with its derivatives Methyl-dibenzofuran and phenyl-dibenzofuran are important derivatives of dibenzofuran, and usually detected in the aromatic fraction of coal, sedimentary rocks, combustion products of municipal solid waste, and crude oil (Yang L et al., Energy Fuels 2017, 31, 3, 2513). The relative importance of their isomers may be used as maturity indicators for sedimentary rocks and crude oils.
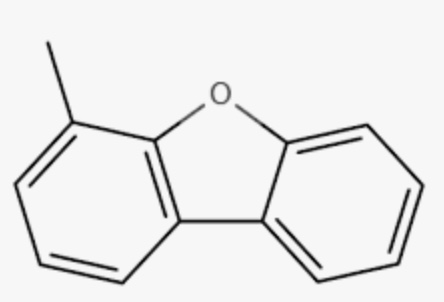
4-Methyl dibenzofuran
4-Phenyl dibenzofuran
B2 – Cyclic hydrocarbons with nitrogen-heterocycle
These cyclic hydrocarbons include heterocycle(s) containing nitrogen.
Quinolines derivatives. The core of quinolines is the 1-azanaphthalene nucleus. The simplest one is quinolin (or an isomer isoquinoline). That compound was first extracted in 1834 by a German chemist FF Runge, from coal tar. Quinoline is used to make dyes, to prepare hydroxyquinoline derivatives and niacin (nicotinic acid or vitamin B3).
Quinolin
That compound is rarely found in living material but is present, as its derivatives, in the plant Rutales and even in some insects. Quinolin is also present in some insects, as phasmids, where it plays a role against predators. The unique quinoline scaffold has many advantages in the discovery of new pesticides and can provide innovative and feasible solutions for the discovery of new pesticides (Cai Q et al., J. Agric Food Chem 2024, 72, 22, 12373).
Several of its derivatives are useful in diverse applications (as 8-hydroxyquinoline). That compound has been identified in the root exudates of Centaurea diffusa (diffuse knapweed), which had not been previously reported as a natural product (Vivanco JM et al., Ecol Lett 2004, 7, 285). Authors found that Centaurea diffusa suppressed the growth of North American species by about 70% more than it suppressed the growth of Caucasian species.
8-Hydroxyquinoline
Quinine is the most famous derivative of quinolin. It has been isolated in 1820 from the bark of a Cinchona tree or from Remijia. Its synthesis was accomplished in 1944 by the American chemist R.B. Woodward.
Initially, quinine is a medication used to treat malaria but this has become less common due to life-threatening side effects.
 Quinine
Quinine
A more recent derivative, hydroxychloroquine, is a medication used for the prevention and treatment of certain types of malaria, rheumatoid arthritis, and lupus.
Isoquinoline is an individual chemical specimen but is used also for the family of many thousands of natural plant alkaloids. It is a structural isomer of quinoline. Both are benzopyridines. Isoquinoline was first isolated from coal tar by Hoogewerf S et al. in 1885.
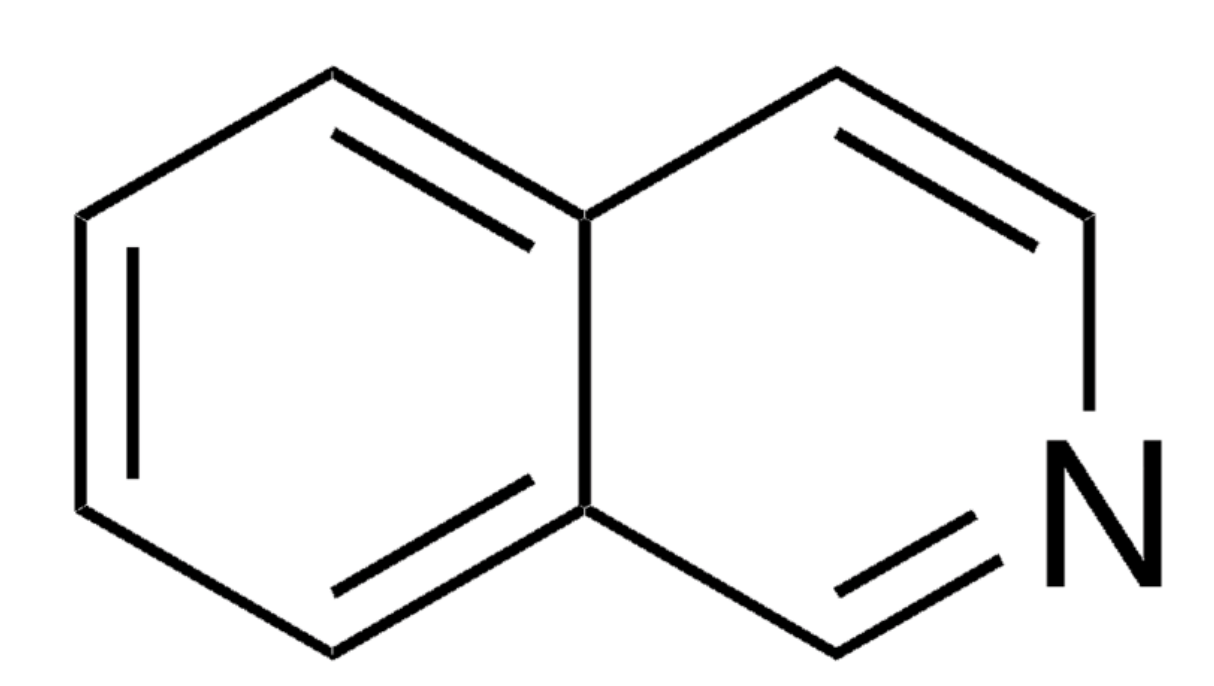
Isoquinoline
1-Benzylisoquinoline is the structural backbone found in many naturally occurring alkaloids such as papaverine.
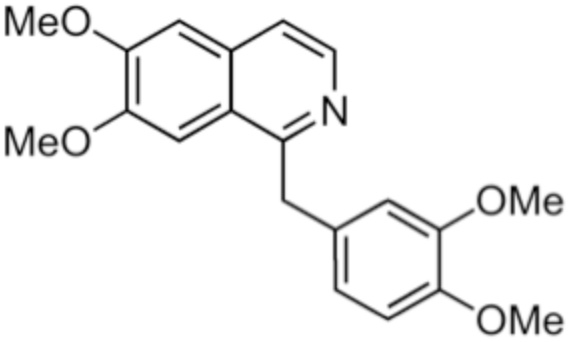 Papaverine
Papaverine
These alkaloids are natural products, chemically derived from isoquinoline, form the largest group among the alkaloids. They can be further classified based on their different chemical basic structures. The most common structural types are the benzylisoquinolines and the aporphines. According to current knowledge, a total of about 2500 isoquinoline alkaloids are known nowadays, which are mainly formed by plants (Wikipedia).
The bacteria Pseudomonas aeruginosa was shown to produce, besides an alkyl homoserine lactone, new cell-to-cell signal molecules (quorum sensing system). This molecule was determined to have a 4-quinolone base structure with an alkyl chain (2-heptyl-3-hydroxy-4-quinolone) and therefore has been designated as the Pseudomonas quinolone signal (Pesci E et al., Proc Natl Acad Sci 1999, 96, 11229). Similar compounds have been previously described for their antimicrobial activities (Hays EE et al., J Biol Chem 1945, 149, 725). It was found that this molecule controlled the expression of genes encoding for the major virulence factors. The maximal quinolone production occurs at the end of the exponential growth phase, supporting the hypothesis that it acts as a secondary regulatory signal for a subset of quorum sensing-controlled genes.
2-Heptyl-3-hydroxy-4-quinolone
Later, another analogue was isolated from the same bacteria, 3,4-dihydroxy-2-heptylquinoline, which could be the direct precursor of the previous quinolone and, likely, the message molecule involved in cell-to-cell communication (Deziel E et al., Proc Natl Acad Sci 2004, 101, 1339). A review of that type of quorum sensing may be read for further information (Dubern JF et al., Mol Biosyst 2008, 4, 882).
In contrast with Pseudomonas quinolone, Burkholderia spp produce several hydroxy-quinolines with a methyl group in position 3 and a hydroxyl group in position 4 and various alkyl chains (Vial L et al., J Bacteriol 2008, 190, 5339). It was later shown that these quorum-sensing signals control the bacterial synthesis of antibiotics (Duerkop BA et al., J Bacteriol 2009, 191, 3909).
A new quinoline-2-carboxylic acid, 4-hydroxy-6-methoxyquinoline-2-carboxylic acid (6-methoxy-kynurenic acid), has been isolated from Ephedra pachyclada. Kynurenic and 6-hydroxykynurenic acids, previously reported from plants, were also isolated from Ephedra (Starratt AN et al., Phytochemistry 1996, 42, 1477).
Cryptolepine is an alkaloid with antimalarial and cytotoxic properties but because of its toxicity, it is no more considered appropriate for use as an anti-malarial drug in humans. It can be found in the roots of the West African plant, Cryptolepis sanguinolenta (Appocynaceae). It remains a herbal product used in the treatment of malaria in the Ashanti region, Ghana. Cryptolepine and its halogenated derivatives were discovered as multitarget inhibitor against all of four insect chitinolytic enzymes from Ostrinia furnacalis (Xie H et al., J Agric Food Chem 2025, 73, 21, 12563).
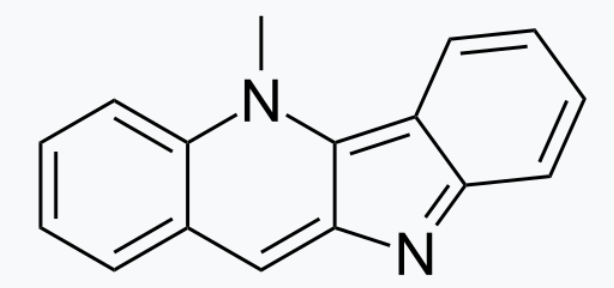 Cryptolepine
Cryptolepine
Compounds related to quinolines, the benzoxazinones, are present as inactive glucosides (phytoanticipines), mainly in Gramineae (rye, wheat, corn) (Niemeyer HM, Phytochemistry 1988, 27, 3349). They are sometimes described as cyclic hydroxamic acids. In rye, the principal compound is the glucoside of DIBOA (2,4-dihydroxy-1,4-benzoxazin-3-one), in wheat and corn, it is the glucoside of the methoxylated form, DIMBOA (2,4-dihydroxy-7-methoxy-1,4-benzoxazin-3-one).
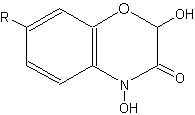
Benzoxazinones
(R = OCH3 : DIMBOA , R = H : DIBOA)
These glucosides are hydrolyzed during the plant infection by fungi or bacteria or after insect attacks. The benzoxazinones aglycones, and their breakdown products (phenoxazinones, acetamides, and malonamic acids), behave like antifungal, antibacterial and also insecticidal substances (allelopathy). These aspects of allelopathy have been reviewed for the sustainable weed control in rye fields (Schulz M et al., J Chem Ecol 2013, 39, 154).
Investigations in genetic engineering are performed to induce the production of these protective agents in other crop plants.
Isoquinolines find many applications, including : anesthetics (dimethisoquin), antihypertension agents (quinapril), antiretroviral agents (saquinavir), and vasodilators (papaverine).
Several isoquinolines have been isolated from Zanthoxylum nitidum (or Shiny-leaf prickly-ash) and display antiproliferative effects against human cancer cell lines (Qin F et al., Phytochemistry 2023, 205, 113476).
Berberine is a molecule belonging to the group of benzylisoquinoline alkaloids found in such plants as Berberis, Mahonia, Hydrastis, Xanthorhiza, Phellodendron, Coptis, and Eschscholzia. Due to berberine’s strong yellow color, Berberis species were used to dye wool, leather, and wood. Under ultraviolet light, berberine shows a strong yellow fluorescence, so it is useful in histology and biochemistry to detect lipids on chromatograms.
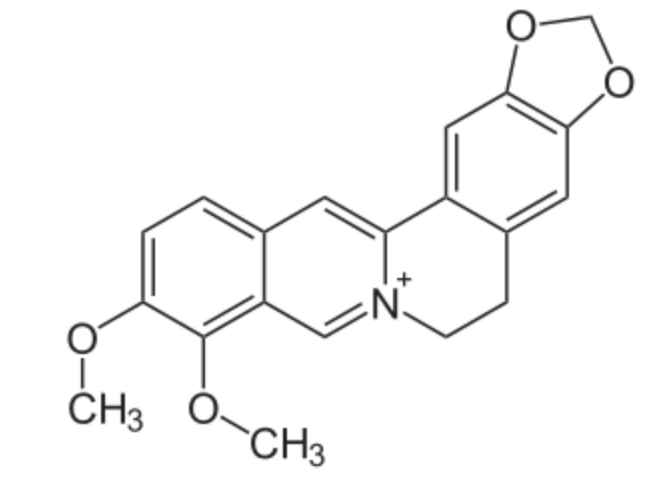
Berberin
Berberine was used in China as a folk medicine and is still a common over-the-counter medication for gastrointestinal infection in China. Studies have been conducted to determine if berberine may affect diabetes. Some research has shown that it may be used against some Staphylococcus aureus infection or diarrhea caused by enterotoxigenic E. coli. Recently, it has been reported that berberine helps in reducing cholesterol and lipid accumulations in both the plasma and in the liver (Doggrell SA, Expert Opin Investig Drugs 2005, 14, 683).
Unfortunately, there is insufficient evidence to conclude that berberine is safe or effective for any condition (Wikipedia).
Sophocarpine is one of the significant alkaloid extracted from the traditional herb medicine Sophora flavescens which has many pharmacological properties such as anti-virus, anti-tumor, anti-inflammatory. Sophocarpine inhibits the growth of gastric cancer cells inducting autophagy and apoptosis. It has been demonstrated to have also anti-tumor activity in various cancer cells, including hepatocellular carcinoma, prostate cancer and colorectal cancer. Furthermore, this molecule has a neuroprotective effect against llutamate-Induced HT22 cell cytotoxicity (Wang T et al., J Oleo Sci 2024, 73, 359).
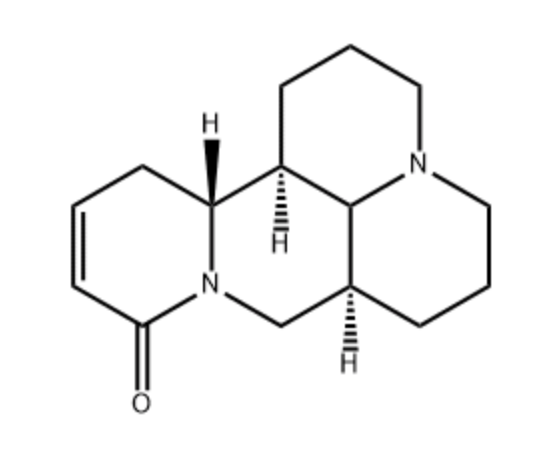 Sophorocapnine
Sophorocapnine
Methyl-Fascaplysin is one of the most powerful marine-derived compounds that produce in vitro neuroprotective effects. Fascaplysin is a extensively active benzoyl-linked β-carboline alkaloid initially extracted from the Fijian sponge Fascaplysinopsis; this molecule showed antitumoral and antioxidative effects (Naveen kumar S et al., Front Lab Med 2018, 2, 41). A study reported that fascaplysin molecule inhibits acetylcholinesterase, implying that this molecule may have positive effects on brain (Manda S et al., Eur J Med Chem 2016, 107, 1).
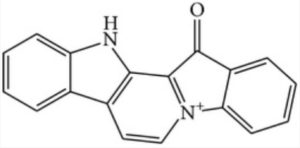
Fascaplysin
B3 – Cyclic hydrocarbons with nitrogen and oxygen heterocycle
Oxazoline is a five-membered heterocyclic organic compound with the formula C3H5NO. It is the parent of a family of compounds called oxazolines. Three structural isomers of oxazoline are possible depending on the location of the double bond (2-, 3-, 4-oxazoline). Several studies have reported that the addition of water to many dry food products, such as chocolate, cornflakes and malt, results in the release of a large amount of Strecker aldehydes.
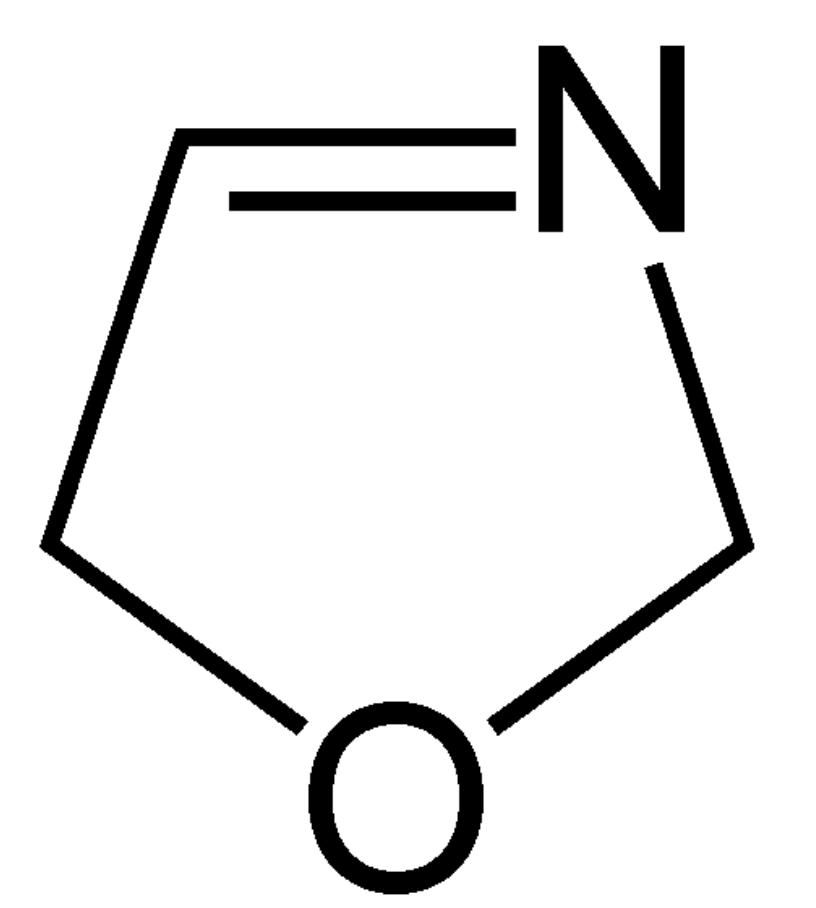 3-Oxazoline
3-Oxazoline
3-Oxazolines have been proposed as precursors, present in cocoa, which hydrolyze to form Strecker aldehydes which are key aroma compounds in roasted food products, such as chocolate, coffee, malt, and bread. The presence of four 4,5-dimethyl-3-oxazolines was detected in cocoa and chocolate (Spooner HG et al., J Agric Food Chem 2025, 73, 15259).
DISPERSIVE LIQUID-LIQUID MICROEXTRACTION
Lire la suiteDevenez membre et participez au développement de la Lipidomique au XXIème siècle.
S'inscrire