
These compounds form the simplest form of lipids, they contain only carbon and hydrogen. They may be divided into aliphatic hydrocarbons with a carbon chain which may be linear (normal), branched, saturated (alkanes) or unsaturated (alcenes, alkynes), cyclic hydrocarbons and carotenoids. Several hydrocarbons may be substituted with oxygen-containing groups and may contain oxygen or nitrogen in one of their carbon rings.
These organic compounds include :
![]()
These molecules are found mainly in petroleum but living organisms, eukaryotic or prokaryotic, contain frequently hydrocarbons which are directly derived from fatty acids. They are known to be present in living matter since 1892 when Shall C (Chem Ber 1892, 25, 1489) identified undecane in ants, and Etard A (C R Acad Sci Paris 1892, 114, 364) identified eicosane in Bryonia dioica.
Those found in petroleum (and in the environment) are considered as mineral oil hydrocarbons or mineral oil products containing 10 to about 50 carbon atoms. Crude mineral oils are by far the predominant source of these compounds, but equivalent products can be synthesized from coal, natural gas or biomass. Mineral oils are complex mixtures of hydrocarbons, principally of straight and branched open-chain alkanes (paraffins) but also largely alkylated cycloalkanes (naphthenes) and aromatic hydrocarbons including mainly alkyl-substituted poly aromatic hydrocarbons.
Decane (C10H22), undecane (C11H24), and dodecane (C12H26) were detected in a Martian sample at the tens of pmol level and may originate from long-chain carboxylic acids. The provenance and distribution of these molecules are of high interest in the search for potential biosignatures on Mars (Freissinet C et al., PNAS, March 24, 2025).
Hydrocarbons derived from fatty acids (i.e. saturatdes alkanes and unsaturated alkenes) are ubiquitous in plants and insects where they often represent a major part of cuticular waxes and play an essential role in preventing water loss from the organisms to the dry terrestrial environment. In several insects, specific cuticular alkenes also act as sex pheromones. Occurrence of alkanes or alkenes has also been reported in various microorganisms (Ladygina L et al., Process Biochem 2006, 41, 1001). For example, synthesis of hydrocarbons is widespread in cyanobacteria (Coates RC et al., PLoS One 2014, 9:e85140), and it is thought that cyanobacterial alka(e)nes contribute significantly to the hydrocarbon cycle of the upper ocean. It has been demonstrted that several microalgae have the ability to convert C16and C18 fatty acids into alkanes and alkenes by a new, light-dependent pathway (Sorigué D et al., Plant Physiol 2016, 171, 2393). The decarboxylation of fatty acids is initiated through electron abstraction from the fatty acid by the photoexcited FAD with a quantum yield >80%, the enzyme complex was named fatty acid photodecarboxylase, thus it may be useful in light-driven, bio-based production of hydrocarbons (Sorigué D et al., Science 2017, 357(6354):903). Alkanes and alkenes of various chain lengths are important targets for biotechnology as they are major components of gasoline (mainly C5-C9 hydrocarbons), jet fuels (C5-C16), and diesel fuels (C12-C20).
These lipids are distinct from the terpenoid hydrocarbons. They have usually a straight chain of up to about 36 carbon atoms but may also be branched, with one or more methyl groups attached at almost any point of the chain. Usually, the methyl group is near the end of the chain (iso or anteiso). They are either saturated or unsaturated (mono or diunsaturated). In contrast with the diversity of methyl-branched alkanes found in insect species, n-alkanes predominate in plants. Among the least polar components of plant surface lipids hydrocarbons with the odd number carbon chains (C15 up to C33) are predominant.
Allenic hydrocarbons, such as 9,10-tricosadiene, 9,10-pentacosadiene, and 9,10-heptacosadiene were isolated from Australian insects (melolonthine scarab beetles) (Dembitsky VM et al., Prog Lipid Res 2007, 46, 328).
Many microalgae contain the highly unsaturated alkene n-C21:6 formed by decarboxylation of the 22:6n-3 fatty acid (Lee RF et al., Phytochemistry 1971, 10, 593). A few species also contain the n-C21:5 alkene (Volkman et al., Org Geochem 1994, 21, 407). Several microalgae were shown to contain long-chain unsaturated alkenes from 19 to 38 carbon atoms and one to four double bonds (review in Volkman JK et al., Org Geochem 1998, 29, 1163).
Diverse mono-, di-, and tri-unsaturated alkenes were detected in eight lichen species isolated in Japan. It was found that the six lichens containing green algal photobionts were distinguished by the presence of 1,8-heptadecadiene, 6,9-heptadecadiene, and 8- and 7-heptadecenes. On the other hand, 1-octadecene, 4-octadecene, and 5-nonadecene were the major alkene components of the two lichens with cyanobacterial photobionts (Ikeda MA et al., Phytochemistry 2021, 189, 112823). These differences in characteristics could be attributed to phylogenetic differences in the photobionts that comprised the lichens, indicating that the alkene composition could be used for lichen chemotaxonomy (Ikeda MA et al., Organic Geochemistry 2023, 179, 104588).
Hydrocarbons are found at the outer surface in higher plant leaves. As an example, C27, C29, and C31 n-alkanes are the most abundant (from 11 to 19%) in needle wax of the Pinaceae Picea omorika (Nikolic B et al., Chem Nat Compounds 2009, 45, 697). In general, long chain n-alkane abundances (i.e. n-C25 and above) are typically higher in angiosperm trees and shrubs than in many gymnosperms, and more specifically, conifers (except Araucariaceae, Podocarpaceae, Taxaceae, and the Cupressoideae and Callitroideae groups within Cupressaceae which have n-alkane concentrations similar to those of angiosperms). The factors that influence the concentration of plant biomarkers and their carbon isotope composition and their use to understand climate, ecosystem, and carbon cycling in the geologic past have been reviewed (Diefendorf AF et al., Org Geochem 2017, 103, 1).
Environmental parameters such as temperature and humidity can affect the composition of higher plant leaf hydrocarbon wax. The abundance and distribution of long-chain n-alkanes in sediments have been proposed as chemical indicators reflecting climate change. Thus, it has been shown that aridity specifically affected the concentration and distribution of n-alkanes in Acacia and Eucalyptus sampled in the North of Australia. Their n-alkane concentration increased by a factor of ten from the sea to the dry center of Australia, but Acacia-alkanes decreased in average chain length towards the arid center of Australia, whereas Eucalyptus average chain length increased under arid conditions. (Hoffmann B et al., Org Geochem 2013, 62, 62).
Field bioassays enabled the discovery of alkanes as bee sex pheromones and orchid attractants. Thus, C21 to C27 alkanes elicited bee pollinator approaches, landings and attempted copulation (Bohman B et al., Curr Opin Plant Biol 2016, 32, 37). The contribution of hydrocarbon to orchid pollination has been reviewed (Perkins J et al., Nat Prod Rep 2023,40, 819).
Its has been demonstrated that in the honey bee, the colony-specific cuticular hydrocarbon profile completes its maturation in foragers via a sequence of stereotypic age-dependent quantitative and qualitative chemical transitions, which are driven by environmentally-sensitive intrinsic biosynthetic pathways (Vernier CL et al., Elife 2019, 8:e41855). Later, authors were showing that differences in the chemical profiles of individuals from different social insect colonies are primarily driven by community-level variations in microbiota across colonies (Vernier CL et al., Sci Adv 2020, 6/42, eabd3431).
They are also abundant at the outer surface of insects and several marine organism. They are thought to serve as a barrier to water influx in the organism, to act as sex attractants (or anti-aphrodisiacs), to affect the absorption of chemicals and microorganisms. Wild populations of Drosophila melanogaster use several cuticular hydrocarbons (mainly 7,11-heptacosene) as sexual pheromone (Cobb M et al., Anim Behav 1990, 39, 1058). The roles of hydrocarbons in the recognition systems of insects has been reviewed (Singer TL, Amer Zool 1998, 38, 394). The blend of linear and branched hydrocarbons from 22 to 34 carbon atoms found on the cuticle of various species of the Coleoptera genus Chrysochus has been shown to mediate mate choice and sexual isolation (Petereson MA et al., Chemoecology 2007, 17, 87). The 7-methyltricosane is a male-predominant cuticular hydrocarbon used as a contact pheromone in the western flower thrips Frankliniella occidentalis (Thysanoptera) (Olaniran OA et al., J Chem Ecol 2013, 39, 559). 5-Methylheptadecane has been identified as the sex pheromone of Leucoptera spartifoliella appearing as a highly specific biological control agent for the Scotch broom, Cytisus scoparius (El-Sayed AM et al., J Chem Ecol 2024, 50, 874).
Several hydrocarbons are produced as alarm pheromones by the Dufour’s gland in the ants (Regnier F E et al., J Insect Physiol 1968, 14, 955). Five hydrocarbons have been described, undecane, tridecane, pentadecane, 2-tridecanone, 2-pentadecanone. During the act of stinging, formic acid and hydrocarbons are discharged simultaneously from these glands in fine droplets. These hydrocarbons act also as spreading agents for formic acid. 9-Tricosene (23 C) is found in the web of some male spiders (Pholcidae) (Schulz S, J Chem Ecol 2013, 39, 1).
Cuticular hydrocarbons have proved to be useful for identifying insect species and differentiating populations. In combination with cuticular hydrocarbons, isoprenoid soldier defensive secretions have been used in some termite species for chemotaxonomic analyses. Thus, analyses have shown that the hydrocarbon profiles of French populations of subterranean termites, Reticulitermes flavipes, were closer to termite populations from Louisiana than to those from Florida (Perdereau E et al., J Chem Ecol 2010, 36, 1189). In ants (Formica exsecta), the chemical profile has been extensively studied across Europe, demonstrating a hydrocarbon profile, composed mainly of homologous series dominated by three n-alkanes (C23, C25, and C27) and three (Z)-9-alkenes (C23:1, C25:1, and C27:1) (Martin SJ et al., J Chem Ecol 2013, 39, 1415). Only the (Z)-9-alkenes have been shown to act as nest-mate recognition cues, with changes in n-alkanes corresponding with task differences. Similar results have been described in other ants and in honeybees. It has been shown that n-alkanes and (Z)-9-alkenes respond to environmental factors, but often in different ways, indicating that their production is controlled by different genetic pathways. Studies of phenotypic variations in various ant population support the primary role of (Z)-9-alkenes as recognition cues and that of n-alkanes, and other cuticular lipids, as anti-desiccants.
Analyses of cuticular lipids of adult Schistocerca shoshone have revealed that insects had the same hydrocarbons, but their relative abundance varied between populations and, within a locality, remained more or less constant over time. It was observed that the cuticular lipids of insects from a population living in an area with high summer temperatures included higher proportions of n-alkanes than those of insects from a less extreme environment (Chapman RF et al., Biochem System Ecol 1995, 23, 383).
Field bioassays enabled the discovery of alkenes as bee sex pheromones and orchid attractants. Thus, (Z)-9, (Z)-11 and (Z)-12 alkenes (C25, C27, C29) elicited bee pollinator approaches, landings and attempted copulation (Bohman B et al., Curr Opin Plant Biol 2016, 32, 37). Additive bioassays indicated the importance of the alkene double bond position for controlling pollinator preference.
Several hydrocarbons (octane, nonane, dodecane, hexadecane…) belong to aroma compounds which are found in environmental or food systems (see the website Flavornet).
Several chemical compounds derived from petroleum distillation and refining belong to what is named “mineral oil saturated hydrocarbons”. Among them, some can enter food in many ways (environment, lubricants, food additives, migration from contact materials). They are able to be accumulated in the liver and the lymphoid system, but do not seem to present a public health risk at current levels.
Various hydrocarbons are present in the photosynthetic prokaryotes but in low concentrations. Most species have from 15 to 20 carbon atoms, heptadecane being by far the most predominant in all species. In some species, mono- or di-unsaturated chains (alkenes) were found, in others (Cyanobacteria) methyl-branched alkanes are present.
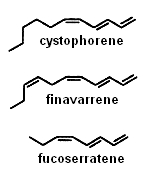
Several alkenes with 8 or 11 carbon atoms and 3 or 4 double bonds play a role in algae gamete attraction (pheromones) : cystophorene in Cystophora sp, finavarrene in Ascophyllum sp and Sphaerotrichia sp, fucoserratene in the brown seaweed Fucus serratus and in the freshwater diatom Asterionella formosa (Bacillariophyceae).
During the investigation of marine aroma components in the brown algae Sargassum thunbergii, characteristic volatile hydrocarbons have been detected. Thus, (6Z,9Z,12Z,15Z,18Z)-1,6,9,12,15,18-henicosahexaene and (6Z,9Z,12Z,15Z-1,6,9,12,15-henicosapentaene have been analyzed (Lu SJ et al., J Oleo Sci 2018, 67, 1463).
Hydrocarbons are also formed as products of fatty acid cleavage during peroxidation processes. Alkanes as well as alkenes appear during hydroperoxide decomposition.
Long-chain (>C25) n-alkyl hydrocarbons are among the most important biomarkers for reconstructing past environmental and climatic conditions. For example, long-chain n-alkanes are considered biomarkers for terrestrial inputs to remote sediments in the South Pacific of the Southern Ocean through long-range aeolian dust transport and in ArabianSea. Nevertheless, it has been demonstrated that long-chain n-alkyl lipids can originate from aquatic microbial sources as they are present in Antarctic lakes, where no vascular plants are present (Chen X et al., Org Geochem 2019, 138:103909).
Chlorinated hydrocarbons are industrial chemical compounds produced in high volume since the 1930s. They are mainly used as plasticisers, flame retardants and lubricants. The main constituents based on n-alkanes, some of them being also based on branched alkanes. Depending on the carbon chain length, mixtures are classified as short-chain (C10–13), medium-chain (C14–17) or long-chain (C18–30) chlorinated hydrocarbons. The chlorine content may be between 30 and 70 % by mass.
Short and medium-chain chlorinated hydrocarbons have a very high persistence, bioaccumulation, very high bioaccumulation and toxicity. It is therefore necessary to assess the exposure of populations to these contaminants. Human exposure to these molecules through mainly food, but dust ingestion, inhalation and skin have already been reported. In France, daily intakes have been estimated to 7.9 and 100 ng per kg of body weight per day for ∑(C10–13) and ∑(C14–17), respectively (Amouro C et al., Food Chem 2025, 488, 144746).
Chlorinated hydrocarbons with more than 15 carbons have been reported in the leaf waxes of halophytic Chenopodiaceae (Grossi V et al., Phytochemistry 2003, 63, 693). Occurrence of long chain chloroalkenes and chloroalkanes with 30 to 36 carbons have been reported in lake sediments from the Galapagos Islands (Zhang Z et al., Org Geochem 2013, 57, 1). The precursor of these long chain lipids remains yet an enigma.
Among hydrocarbons, a variety of forms are described (saturated or unsaturated):
The normal paraffins : their general formula is CH3(CH2)nCH3 (n= 6 – 40 or greater).
Paraffins may have branched chains :
– one methyl group (monobranched), iso-branched hydrocarbons (methyl group on the second carbon) or anteiso-branched hydrocarbons (methyl group on the third carbon)
(as below)
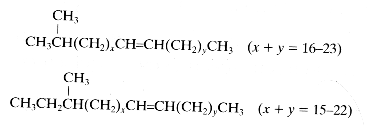
Among the almost 1,000 cuticular hydrocarbons present in ant species, about 200 monomethylakanes and 600 dimethylakanes are used for chemical communication (Martin S et al., J Chem Ecol 2009, 35,1151). Odd chain lengths and positions of methyl at odd carbon numbers are far more numerous than even chain-length compounds. That chemical recognition, fundamental in insect societies, is known for over 100 years in ants and was shown to be based on antennal detection of non-volatile compounds found on cuticle (Fielde AM, PNAS 1901, 53, 521). Since 1987, the nest-mate discrimination systems in several ant species are known to be based on hydrocarbons (Bonavita-Cougourdan et al., J Entomol Sci 1987, 22, 1).
In longhorned beetle, Mallodon dasystomus (Coleoptera, Cerambycidae), cuticular hydrocarbon profiles of females contained 13 compounds that were not present in profiles of males. Among the female-specific compounds, two co-dominant methylbranched alkanes, 2-methylhexacosane and 2- methyloctacosane, are contact pheromes and accounted for 17% of the total hydrocarbons (Spikes AE et al., J Chem Ecol 2010, 36, 943).
– several methyl groups (multibranched), one for each unit deriving from the isoprene formula : CH2=C(CH3)-CH=CH2. Hydrocarbons formed of isoprene units belong to the large group of terpenes.
This chain type is frequently found in several lipid forms, either isolated or combined with other chemical structures. A series of long-chain methylated alkanes (more than 23 carbon atoms), saturated or with one double bond, were identified in settling particles and surface sediments from Japanese lakes and were shown to be produced by planktonic bacteria being thus useful molecular markers (Fukushima K et al., Org Geochem 2005, 36, 311). Laboratory experiments have demonstrated that n-alkanes up to C35 may be formed in the laboratory under hydrothermal conditions (Fischer-Tropsch-type reactions) from formic acid or oxalic acid (Mccollum TM et al., Orig Life Evol Biosph 1999, 29, 153). These results support the theory of the origin of life in hydrothermal systems.
Methoxyalkanes have been identified on bodies or silk of spiders : 1-methoxy-16,20,24,28-tetramethylhentriacontane and 1-methyl-2,24-dimethyloctacosane (Schulz S, J Chem Ecol 2013, 39, 1).
It must be noticed that highly branched and unsaturated (2-5 double bonds) isoprenoids are widespread components in marine sediments (review by Rowland SJ et al., Marine Envir Res 1990, 30, 191; Belt ST et al., Geochim Cosmochim Acta 2000, 64, 3839). The identification of C25 and even of C30 highly branched isoprenoid alkenes in diatoms (Johns L et al., Org Geochem 1999, 30, 1471) have clearly established that they are the source of these compounds found in sediments.
Among the saturated isoprenoids found in geological sediments and oils, the most frequent are pristane (2,6,10,14-tetramethylpentadecane) and phytane (2,6,10,14-tetramethylhexadecane). Both compounds can be generated diagenetically from the phytol side chain of chlorophyll. Pristane may also derive from the side chain of tocopherols while phytane is also generated by Archaea.
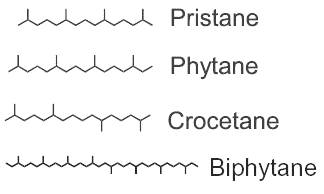
The widespread use of pristane as a biological marker is related to its structural similarity to phytol and its apparent stability, in connection with inability of microorganisms to carry out its anaerobic destruction. pristane is present in photosynthetic organisms, it has been detected in bacteria, algae, and higher plants. Marine sources of pristane include zooplankton, lobster, fish, sharks, sperm whale. Fossil fuels such as coal and petroleum contain this compound. The stable structure persists even in Precambrian rocks and perhaps in extraterrestrial meteorites. The coexistence of microfossils with pristane and phytane in Precambrian rocks is significant to the paleobotanist. Despite this inertness, pristane can be utilized as the sole source of carbon and energy for growth of a coryneform soil isolate ( McKenna EJ et al., PNAS 1971, 68, 1552).
Crocetane is formed by four isoprene units arranged symmetrically around a tail-to-tail linkage (2,6,11,15-tetramethylhexadecane). It was initially synthesized and named by Karrer et al. (Helv Chim Acta 1930, 13, 707). Crocetane is mainly present in oils and in methane-rich sediments, as it was shown to be produced by microorganisms utilizing methane as their carbon source. 2,6,10,15,19-Pentamethylicosane differs from crocetane by the addition of a single isoprene unit, joined head to tail, at one end of the molecule. This compound is present in methane-rich sediment and is likely produced by anaerobic methanotrophs.
Phytane and biphytane, present in sediments and petroleum, are thought to derive from ether-lipids (archaeol, caldarchaeol) of Archaea, the only organisms known to possess such structures.
The separation of straight chain and branched chain alkanes is efficient on a micro scale using the urea complex formation as described for fatty acids (Xu S et al., Org Geochem 2005, 36, 1334). That efficient method is based on the urea inclusion directly on the TLC plates and successive elutions with two solvents to separate straight and branched alkanes.
One important member of isoprenoid polyenes is squalene (C30H50) which is a metabolic precursor of sterols and steroids and classified into the triterpenoids. It is also a component of sebaceous lipids (12-15% of sebum weight) found on human skin. It consists of 6 isoprene units and contains 6 trans double bonds. It was discovered in 1906 in shark oil (Tsujimoto M, J Soc Chem Ind Jpn, 1906, 9, 953). It was suggested that squalene and its peroxidized derivatives (6 hydroperoxysqualene are possible) occurring by UV irradiation have an important role in the occurrence of sunburn, and/or protection from sunburn skin damage (Ohsawa K et al., J Toxicol Sci 1984, 9, 151). It has been shown that hydroperoxysqualene induces expression of genes linked to inflammation but its effect may be lowered by gamma-tocotrienol, a form of vitamin E (Nakagawa K et al., Lipids 2010, 45, 833).
Furthermore, it has been suggested that squalene peroxides may play an important part in the pathology of acne, pityriasis versicolor, and skin aging. There is some evidence that squalene reduces colon cancer (Rao et al., Carcinogenesis 1998, 19, 287) and skin cancer (Owen R et al., Food Chem Toxicol 2000, 38, 647). This activity is likely related to its antioxidant effect (Amarowicz R, Eur J Lipid Sci Technol 2009, 111, 411).
It must be mentioned that if squalene is found in large quantities (from 0.2 to 0.9 g per Kg) in some fish liver oils (shark), it is also found in olive oil (its content may vary from 1 to 40 mg per Kg) where it is used to detect any adulteration. Other vegetable oils contain only traces of this compound (from 0.02 up to 0.3 mg per Kg). Squalene was also found in the epicuticular wax of fruit (grapefruit) and in the hydrocarbon fraction of wheat.
Squalene, and its saturated derivative (squalane) present in skin sebum, are largely used in cosmetics. Squalane is obtained by hydrogenation of the squalene pool isolated mainly from olive oil.
A range of C25 highly branched isoprenoid alkenes were discovered in filtered phytoplankton samples from Arctic waters and from sub-Antarctic waters. These alkenes contained 3–5 double bonds and had structures identified previously from analysis of laboratory diatom cultures.They were also identified in diatoms of the genus Rhizosolenia isolated from seawater collected in the South Atlantic (Belt ST et al., Org Geochem 2017, 110, 65).
It has been shown that presqualene diphosphate, intermediate between farnesyl diphosphate and squalene, carries biological activity in human neutrophiles and serves as a negative intracellular signal preventing superoxide anion generation (Levy BD et al., Nature 1997, 389, 985). An inhibition of phosphatidylinositol 3-kinase in the same cells was also demonstrated (Bonnans C et al., J Exp Med 2006, 17, 203, 857). During that signaling step, squalene diphosphate is transformed into the inactive monophosphate species (Fukunaga K et al., J Biol Chem 2006, 281, 9490).
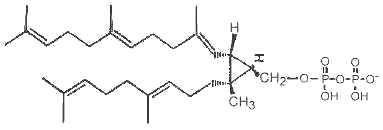 Presqualene diphosphate
Presqualene diphosphate
Two homologous series of trimethylalkanes, the 3,7,11-trimethylalkanes (C34H7), C36H74 and C38H78) and the 4,8,12-trimethylalkanes (C35H72, C37H76 and C39H80) have been described as the major constituents of the cuticular alkanes of the ant, Atta colombica (Martin MM et al., Tetrahedron 1970, 26, 307). Each of these structures combines a reduced polyketomethylene chain and a modified isoprenoid chain, and hence combine structural units from two major metabolic pathways. Hydrocarbons of this type have not been previously isolated from natural sources.
Pheromones with an epoxy ring are biosynthesized from unsaturated hydrocarbons by action of epoxidases specific for one specific double bond. In the case of monoepoxides derived from 3,6,9-trienes, all three kinds of epoxydienes (3,4-epoxy- 6,9-dienes, 6,7-epoxy-3,9-dienes, and 9,10-epoxy-3,6-dienes) have been found in female moths, 9,10-epoxy-1,3,6-triene has been identified as a natural pheromone component from two species in Arctiidae, the fall webworm (Hyphantria cunea) (Tóth M et al., Tetrahedron Lett 1989, 30, 3405) and the mulberry tiger moth (Lemyra imparilis) (Ando T et al., Topics Current Chem 2004, 239, 51). 9,10-Epoxy-(3Z,6Z)-1,3,6-henicosatriene has been identified from a pheromone gland of arctiid species, such as Hyphantria cunea (Yamakawa R et al., J Chem Ecol 2012, 38, 1042).
ALKYNES – In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond or acetylene group. Acetylene compounds or “polyacetylenes” (the latter term is often used) are a general name for a substantial class of natural hydrocarbons, all containing one or more carbon−carbon triple bond functionalities in their molecules. Naturally occurring polyacetylenes feature a wide range of structural diversity and are widespread in plants and animals (Minto RE. et al., Prog. Lipid Res 2008, 47, 233). In the marine environment, they have been isolated mainly from algae and invertebrates, and have displayed a broad array of biological properties, including antifungal activity, antimicrobial activity, HIV reverse transcriptase inhibition, and cytotoxicity. In addition, important ecological roles, such as inducing metamorphosis of ascidians’ larvae, preventing fouling by barnacle larvae, and inhibiting fertilization of starfish gametes, have been ascribed to these metabolites. Polyacetylenes of marine origin, including their chemistry and bioactivity, were reviewed in an important report (Zhou ZF et al., Chem Rev 2015, 115, 1543).
Acetylenes isolated from algae are mostly C-15 metabolites, a group of compounds of great importance. Almost all of them were isolated from the red algae belonging to the Laurencia genus, making them characteristic metabolites of the Laurencia algae. As an example, laurencenyne has been isolated from Laurencia okamurai (Kigoshi H et al.. Tetrahedron Lett 1981, 22, 4729).
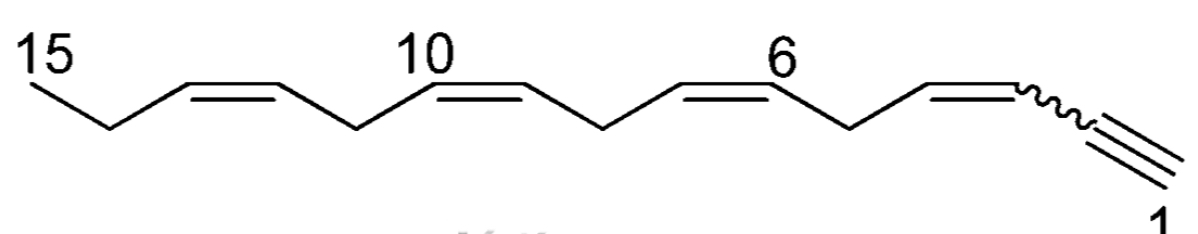
Laurencenyne
Asymmetric poly-acetylenes with long chains ranging from n = 16 to n = 48 are ubiquitous in marine sponges, and they are believed to play a crucial role in the sponge metabolism.
Hydrocarbons may be classified into monocyclic and polycyclic species.
Several chemical compounds derived from petroleum distillation and refining belong to what is named “mineral oil aromatic hydrocarbons”. Among them, some can enter food in many ways (environment, lubricants, food additives, migration from contact materials). Some of them may be genotoxic and thus they can damage DNA in cells and may cause cancer.
Cyclic hydrocarbons (or arenes) are either benzene derivatives or non-benzoids (with other aromatic cycle derivatives). There may be also heteroarenes when they contain furan, pyridine or others nuclei.
One of the simplest monocyclic hydrocarbons is 1-Methylcyclopropene, a synthetic derivative of cyclopropene used as a plant growth regulator. It turns to vapor at temperatures higher than 12°C. It is structurally related to the natural plant hormone ethylene and it is used commercially to slow down the ripening of fruit and to help maintain the freshness of cut flowers. Its mechanism of action involves its tightly binding to the ethylene receptor in plants, thereby blocking the effects of ethylene (ripening of climacteric fruit, opening of flowers, shedding of leaves). Its use has been approved and accepted for use in more than 34 countries including the European Union and the United States.
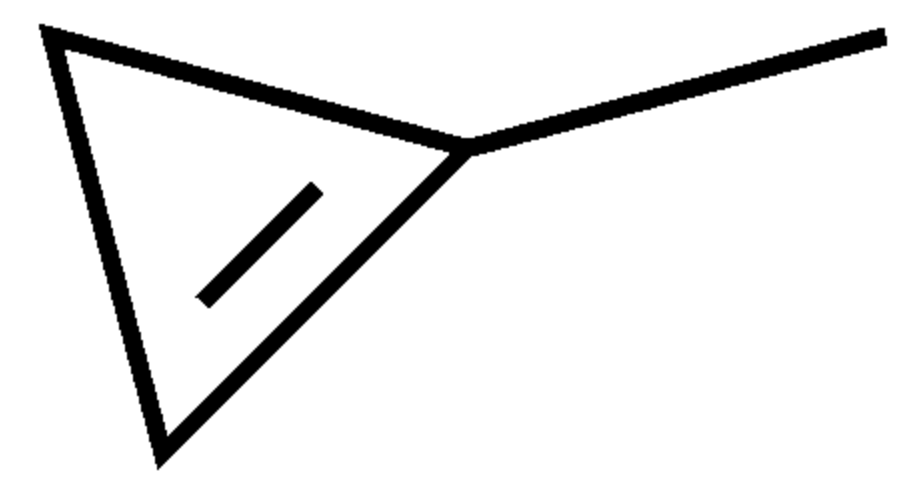 Methylcyclopropene
Methylcyclopropene
Several branched-alkylbenzenes have been described in Archaebacteria such as Thermoplasma and Sulfolobus. They have mostly two methyl groups branched on a saturated chain of 9 to 12 carbon atoms. One of them is shown below.
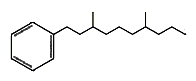
Long chain alkyl benzenes and alkyl toluenes are common con- stituents of crude oils and sedimentary organic matter. Among them, phytanyl benzene (and phytanyl toluene) occurs in mudstones from Permian-Triassic Boundary sections from Spitsberg and Greenland), as well as Western Australia. Despite their ambiguous origin, the differences in their distributions indicate that they can be characteristic of specific microbial communities such as Archaea (Grotheer H et al., Org Geochem 2017, 104, 42).
Phytanylbenzene
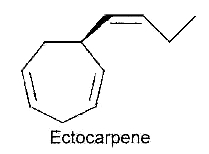
After the first hypothesis of a communication system (chemotaxis) by Thuret MG for the fertilization of brown algae, 117 years were needed to know the structure of the first algal pheromone (Müller DG et al., Science 1971, 171, 815). This compound, ectocarpene, is an unsaturated heptacyclic hydrocarbon found in the brown algae Ectocarpus, Adenocystis and Sphacelaria. It belongs to a large group of chemicals named dictyopterenes. That compound has a fruity scent and can be sensed by humans when the female organs start emitting the substance to attract the male gametes.
Among the numerous dictyocarpenes, dictyopterene A (trans-1-(trans-1-hexenyl)-2-vinylcyclopropane) is naturally present in marine and freshwater environments and in lipids extracted from several species of brown algae such as Dictyopteris (Phaeophyceae) (Jiittner F et al., Limnol Oceanogr 1984, 29, 1322). Dictyopteris is an important group of marine seaweeds and is widely distributed in tropical, subtropical and temperate regions. This genus is known by its richness in chemicals which are in part the source of its characteristic “ocean smell” (Zatelli GA et al., Revista Brasileira de Farmacognosia 2018, 28, 243).
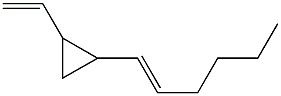 Dictyopterene A
Dictyopterene A
Sarekensane is the first nonterpenoid cuticular hydrocarbon from Collembola that is biosynthesized via the fatty acid pathway, as are insect hydrocarbons, and contains two cyclopropane rings in the chain, not previously reported from arthropods (Möllerke A et al., J Nat Prod 2024, 87, 85).
 Sarekensane
Sarekensane
Several heptacyclic compounds similar to ectocarpene play a role of pheromone in marine brown algae, dityotene in Dictyota sp, desmarestene in Desmarestia sp and Cladostephus sp, lamoxirene in Laminaria sp, Alaria sp and many others.
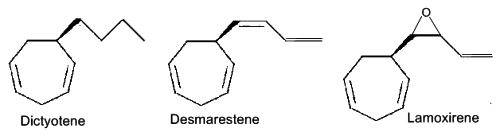
Heptacyclic compounds from brown algae
The C11-hydrocarbon ectocarpene was also detected as component in the odor of ripening mango (Berger RG et al., J Agric Food Chem 1985, 33, 232) but was also shown to be a metabolite in leaves of the Asteraceae Senecio isatideus (Bohlmann F et al., Phytochemistry 1979, 18, 79). Several biosynthetic studies suggest unsaturated fatty acids as precursors of these pheromone hydrocarbons (Pohnert G et al., Nat Prod Rep 2002, 19, 108). A review of hydrocarbon as chemical signals in algal gamete attraction has been released (Boland W, PNAS 1995, 92, 37).
Several parent molecules (linear, tri-, penta- or hexacyclic) with different degrees of unsaturation and chain lengths have been described in various algal species (Pohnert G et al., Nat Prod Rep 2002, 19, 108).
Dehydrozingerone can be obtained from ginger (Zingiber officinale, Zingiberaceae) or through a laboratory-based synthesis procedure. It is known as a feruloylmethane analog of curcumin, but also as a metabolite that contains half of the structure of curcumin. It possesses antioxidant and anti-inflammatory activities that are similar to those of curcumin including a potential anti-prostate cancer inhibitor (Mapoung S et al., Molecules 2020, 25(12), 2737). Results indicated that dehydrozingerone possessed a strong neuroprotective effect through its ability to ameliorate the Parkinson’s disease-like phenotypes in Drosophila model (Setzu MD et al., Biomolecules 2024, 14(3), 273). If validated in mammalian models, it could be considered a promising compound for the development of agents with potent neuroprotective properties.
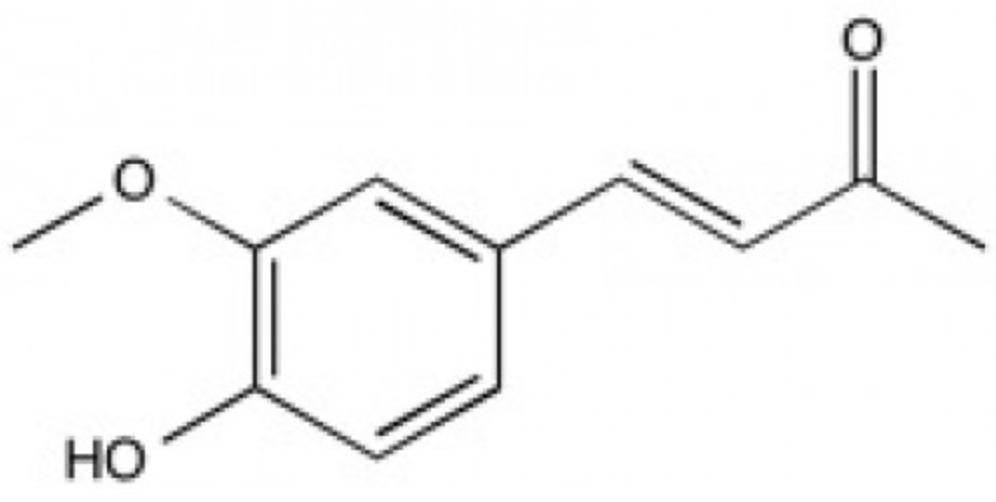 Dehydrozingerone
Dehydrozingerone
Cuminaldehyde or cuminal (4-isopropylbenzaldehyde) is a natural compound with an isopropyl group substituted in the 4-position. It is a constituent of the essential oils of eucalyptus, myrrh, cassia, and mainly cumin (Cuminum cyminum). It has a pleasant smell and is used commercially in perfumes and other cosmetics.
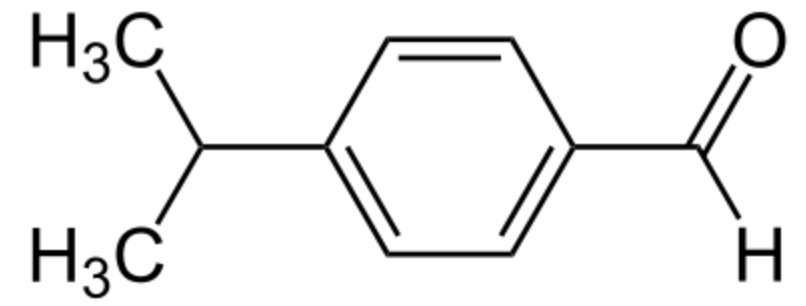
Cuminaldehyde (cuminal)
Phthalates are esters of phthalic acid with different alcohols (methyl, isobutyl, butyl, hexyl, octyl …). The most important form is di-(2-ethylhexyl)-phtalate (DEHP), which accounts for about 50% of the world production of phthalates (Wikipedia) which are used as plasticizers.
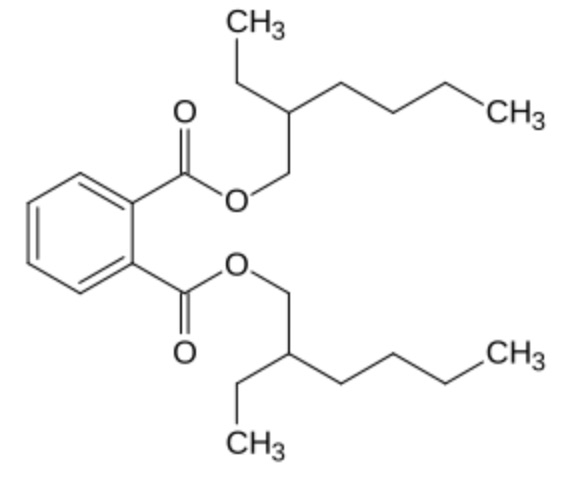
Di-(2-ethylhexyl)-phtalate
Phthalates are mainly used in cosmetics (perfume, hair spray, soap, shampoo …) and in a lot of consumer products made in various plastic substances, therefore they are commonly found in fat-containing foods despite the international decision that they can only be used as plasticizers for plastic material in contact with non-fatty food. Their biodegradation in soil occurs by sequential hydrolysis of the two diethyl chains but that reaction occurs very slowly in an abiotic environment. Studies have suggested that some phthalates affect male reproductive development via inhibition of androgen biosynthesis but it seems more evident that certain high doses of phthalates may cause skeletal malformations after administration in pregnant animals,
A benzyl cyanide derivative has been described in the butterfly Pieris brassicae ( Andersson J et al., J Chem Ecol 2003, 29, 1489). It functions as an anti-aphrodisiac transferred by the male to the female.
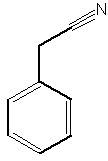
Benzyl cyanide
Phenylethyl isothiocyanate, one of the most important isothiocyanates (R-N=C=S), is present in the cruciferous vegetable watercress (Nasturtium officinale). It is the most potent antioxidant among Brassicaceae, present as glucosinolate gluconasturtiin, which, on hydrolysis, produces phenylethyl isothiocyanate. This biosynthesis takes part in the formation of “mustard oil bomb“, a chemical defense system against herbivory found in members of the cabbage family.
Phenylethyl isothiocyanate exhibits antioxidant and anti-inflammatory, bactericidal), and anti-carcinogenic effects, among other benefits. Recent reviews suggest that it could be a natural nutraceutical or an adjuvant to treat various disorders due to its chemopreventive action and health-promoting effects (Li N et al., Food Chem 2022, 375, 131816).
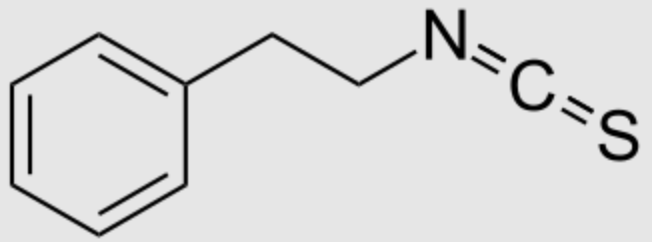
Phenylethyl isothiocyanate
It has been demonstrated that solitary and gregarious locusts are attracted by 4-vinylanisole (or 4-methoxystyrene). Furthermore, 4-vinylanisole could function to both bring solitary locusts into the fold of the swarm, as well as maintain a swarm’s cohesiveness over time (Guo X et al., Nature 2020). The finding could inform new ways of controlling or preventing locust swarms, potentially by attracting the insects with their own scents.
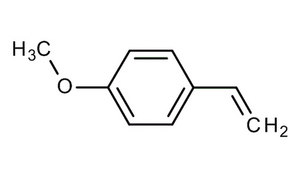 4-Vinylanisole
4-Vinylanisole
The stilbenes (or stilbenoids) form a group of numerous compounds classified in the phenylpropanoids, based on a simple one, stilbene. That compound consists of two phenyl groups linked by a trans ethene double bond. Its name was derived from the Greek word “stilbos”, which means shining. Stilbene is mainly used in manufacture of dyes and optical brightening agents.
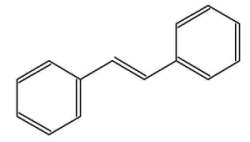
trans-Stilbene
Stilbenes, sometimes classed into the polyphenol group, are present in several vegetal sources. Several forms have been described, differentiated by various substitutions and combinations of hydroxyl or alkoxyl groups. Some stilbenes are glycosylated. Thus, 2,3,5,4′-tetrahydroxystilbene 2-O-b-D-glucoside, the major bioactive compound from Polygonum multiforum, can efficiently inhibit the formation of advanced glycation end products (Lv L et al., J Agric Food Chem 2010, 58, 2239). A comprehensive review of the source plants, chemistry, biosynthesis, pharmacology, application of stilbenes have been reviewed (Teka T et al., Phytochemistry 2022, 197, 113128).
Studies on the phytochemical composition of different species of rhubarb, Rheum genus (Polygonaceae), were undertaken as early as at the beginning of the 20th century, and provided information on the presence of a variety of stilbenes (review in Kolodziejczyk-Czepas J et al., Phytochem Rev 2021, 20, 589).
A review of the chemistry and the biology of natural stilbenes may be consulted (Zhou L et al., Nat Prod Rep 2025, 42, 359). A review focused to selected bioactive stilbenes as potential chemopreventive agents for colorectal cancer with a focus on their modulatory mechanisms of action, especially in targeting alterations in DNA methylation machinery (Fialková V et al., Nutr Cancer 2024, 76, 760).
Stilbenes belong to the naturally synthesized plant phytoalexins, produced de novo in response to various biotic and abiotic stressors. The importance of stilbenes in plant resistance to stress and disease is of increasing interest (review in Gao Q et al., J Agric Food Chem 2024, 72, 7655).
Diethylstilbestrol, a stilbene derivative, (E)-11,12-diethyl-4,13-stilbenediol, is also classified in the phenylpropanoids group. It is a nonsteroidal estrogen which was used for a variety of indications, including pregnancy support for women with a history of recurrent miscarriage, hormone therapy for menopausal symptoms and estrogen deficiency in women. Today, it is only used in the treatment of prostate cancer and less commonly breast cancer. It has been demonstrated it has the potential to cause a variety of significant adverse medical complications, including transgenerational effects, and thus it was largely discontinued.
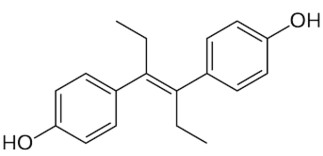 Diethylstilbestrol
Diethylstilbestrol
Resveratrol (3,4′,5-trihydroxystilbene), which belongs to the group of phenylpropanoids, is the most studied because of its presence in grapes and wine and some berries (blueberries, Vaccinium) and its potential pharmacological properties (anti-cancer, antiviral, neuroprotective, anti-aging, and anti-inflammatory). To date, there is no high-quality evidence that resveratrol improves lifespan or has a substantial effect on any human disease (wikipedia).
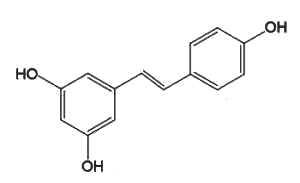
Resveratrol
Some studies have suggested that resveratrol has therapeutic properties in mental disorders, such as major depressive disorder, bipolar disorder, Alzheimer’s disease, and autism (Menegas S et al., J Nutr Biochem 2023, 121, 109435). A systematic review providing an unbiased conclusion on the therapeutic effectiveness of resveratrol in Alzheimer’s disease may be consulted (Azargoonjahromi A et al., Nutr Metabol 2024, 21). As it has been reported, the protective benefits of resveratrol seems to be in preventing the risk of Alzheimer’s disease induced by proinflammatory agents (Bartra C et al., Antioxidants 2024, 13, 177). It has been demonstrated that short chain fatty acid esters enhanced the stability and bioactivity of resveratrol and could be used as a functional food ingredient in processed foods or as dietary supplements to promote health (Huang YN et al., Nutrients 2024, 16, 4076). The effects of substitution sites and acyl chain length on antioxidant capacity and bioaccessibility of high-active resveratrol monoesters in vitro have been examined (Li D et al., Food Chem 2025, 480, 143845).
It has been determined that resveratrol is used by plants as a defensive element (they are named phytoalexins). Thus, grape vine attacked by mildew is able to product resveratrol which can be transformed into glycosylated or dimer compounds. The resistance of some cultivars seems to be related to the toxicity of the derivative (Pezet R et al., Physiol Mol Plant Pathol 2004, 65, 269). Similar observations were reported for conifers.
A resveratrol derivative, oxyresveratrol (4 hydroxy groups instead of 3) is found in the heartwood of Artocarpus lakoocha and in the traditional drug ‘Puag-Haad’ made from it. Interestingly, this compound was found to enhance the anti-cancer effect of cisplatin against epithelial ovarian cancer cells (Thaklaewphan P et al., Biomolecules 2024, 14(9), 1140).
It has been demonstrated that resveratrol monoesters, synthesized by esterifying with lipophilic groups, excel in lipophilicity, antioxidant activity, and bioavailability (Li D et al., Food Chem 15 March 2025, 143845).
Pterostilbene is a stilbenoid chemically related to resveratrol. In plants, it serves a defensive phytoalexin role. It differs from resveratrol by exhibiting increased bioavailability due to the presence of two methoxy groups (increased lipophilic properties). Studies have reported dose-based elevations of low density lipoprotein cholesterol and decreased high density lipoprotein cholesterol. Pterostilbene was evaluated for its protective effects and safety during prolonged use on osteoarthritis (Lee YC et al. J Agric Food Che. 2024, 72, 9150).
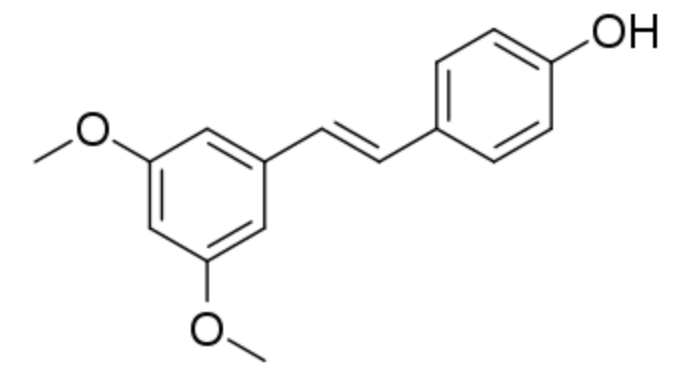 Pterostilbene
Pterostilbene
Dihydro-resveratrol is a dihydrostilbenoid found in wine. It is a metabolite of trans-resveratrol formed in the intestine by the hydrogenation of the double bond of the ethyl group by microflora.
Several more complex derimatives of resveratrol are of biochemical and physiological importance:
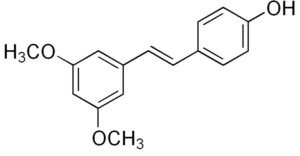 Pterostilbene
Pterostilbene
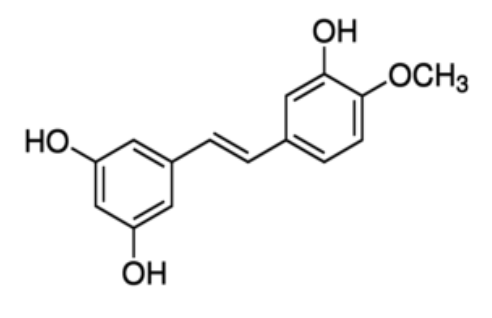 Rhapontigenin
Rhapontigenin
Pharmaceutic industries have developed stilbene derivatives which have estrogenic activity (non-steroidal estrogens) such as diethyl stilbestrol.
Oligostilbenoids (oligo- or polystilbenes) are oligomeric forms of stilbenoids. Some molecules are large enough to be considered polyphenols and constitute a class of tannins (Wikipedia). These lipids, found in Paeonia seeds, have been associated with multiple biological activities (Li XJ et al., 11 Jun 2025 PLOS ONE).
Chalcone is an α,β-unsaturated ketone chemically named (2E)-1,3-Diphenylprop-2-en-1-one. Similar compounds are known collectively as chalcones or chalconoids (Wikipedia). They are widely known bioactive substances, fluorescent materials, and chemical intermediates. They are synthesized in plants as secondary metabolites. Dihydrochalcone is the reduced derivative of chalcone. There are several natural hydrochalcones (aspalathin, naringin dihydrochalcone, nothofagin, phloretin), and some derivatives have attracted attention as drugs. Phloretin is known to inhibit active transport of glucose into cells but that inhibition is weaker than that measured using its glycoside phlorizin (Wikipedia).
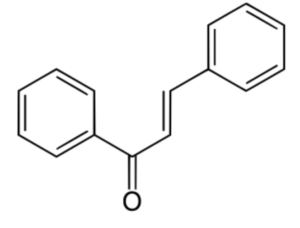 Chalcone
Chalcone
Fatty acid conjugated chalcones have been described as tubulin polymerization inhibitors, thus with potential antiproliferative activity (Mohamed SM et al., J nat Prod 2023, 86, 5, 1150). Decanoic acid conjugates were more potent than longer lipid analogues, with the most active being more potent than the reference tubulin inhibitor, combretastatin-A4 and the anticancer drug, doxorubicin.
Hispidin (6-(3′,4′-dihydroxystyryl)-4-hydroxy-2-pyrone) has been isolated in 1889 from the mushroom Inonotus hispidus (formerly Polyporus hispidus) as a naturally occurring styrylpyrone. Hispidin is a precursor of fungal luciferin, a compound responsible for light emission by luminous mushrooms. Later, a number of other styrylpyrone metabolites from Phellinus and Inonotus have been discovered. Styrylpyrone pigments are common constituents of fungi, mainly those belonging to the Hymenochaetaceae family, including Phellinus and Inonotus (Fiasson J L, Biochem Syst Ecol 1982, 10, 289). They also exist in primitive angiosperm families, including Piperaceae, Lauraceae, Annonaceae, Ranuculaceae and Zingiberaceae, where they have an important role in defense against mechanical wounding or microbial attack. and show potent biological activities, including anti-cancer and sedative effects. Styrylpyrone-class compounds from medicinal fungi Phellinus and Inonotus spp., and their medicinal importance have been reviewed (Lee K et al., J Antibiotics 2011, 64, 349).
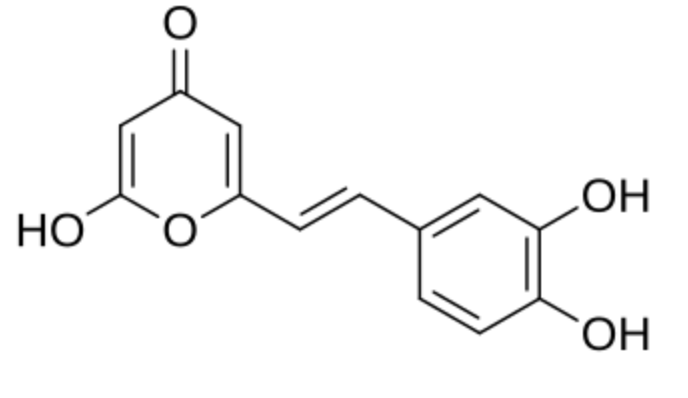 Hispidin
Hispidin
Diarylheptanoids belong to a compound group having phenyl rings at 1,7 positions of n-heptane, such as curcumin and several similar analogues found in the rhizomes of the ginger (Curcuma longa) family (Li J et al., J Nat Prod 2010, 73, 1667). Diarylheptanoids are class of compounds with limited distribution in plant kingdom, found mainly in families Zingiberaceae and Betulaceae.
Their common structure is shown below.
Diarylheptanoid
Curcumin is the principal diarylheptanoid of the Indian spice turmeric, which is a member of the ginger family (Zingiberaceae).
Curcumin
That pigment, which gives the yellow color to turmeric (E100), was isolated two centuries ago, and its structure as diferuloylmethane was determined in 1910 almost hundred years after it was first isolated in 1815 (Milobedzka J et al., Ber Deu. Chem Ges 1910, 43, 2163). Numerous therapeutic activities have been assigned to turmeric for a wide variety of diseases and conditions, they are likely partly related to its strong antioxidant properties (Aggarwal BB et al., Adv Exp Med Biol 2007, 595, 1). An extensive review has been published on the various properties of curcuminoids (Mukherjee S et al., in Herbs and Spices – New Processing Technologies, 2021, Rabia Shabir Ahmad Eds). Curcumin has been shown to inhibit gastric cancer cells through various mechanisms. A review summarized the mechanisms through which curcumin affects gastric cancer in both laboratory and animal studies (Hao M et al., J Funct Foods 2025, 125, 106677).
Plants of the Alnus genus (Betulaceae) have been found to be a valuable source of diarylheptanoids similar to curcumin but with various substituted chemical groups. Some of these compounds have valuable activities against LPS-induced inflammation and could be the source of new anti-inflammatory drugs (Lai YC et al., Phytochemistry 2012, 73, 84).
The reductive metabolites of curcuminoids (tetrahydrocurcuminoids) retain essentially all the useful health-promoting activities of curcuminoids and Japanese researchers have shown that they are more bioavailable than parent curcuminoids (Okada K et al., J Nutr 2001, 131, 2090). These derivatives are found in the plant kingdom, and arise from human metabolism of curcuminoids, but also result from gut microbiota transformation of curcumin. Tetrahydrocurcuminoids are produced chemically by hydrogenation of curcuminoids extracted from the rhizomes of C. longa. The European Food Safety Authority (EFSA) has approved the use of Sabinsa’s C3 Reduct®, a mixture of the most prominent reduced metabolites, for the target population at 140 mg/day.
 Tetrahydrocurcumin
Tetrahydrocurcumin
Polybrominated biphenyl ethers such as 2-(2′,4′-dibromophenyl)-4,6-dibromophenol are characteristic secondary metabolites of the dictyoceratid marine sponge Dysidea herbacea, common in shallow waters of the tropical Pacific Ocean and may represent as much as 12% of the dry weight (Unson MD et al., Marine Biology (1994) 119:1). The production of that compound was shown to be due to the cyanobacterium, and not to the sponge or symbiotic heterotrophic bacteria.
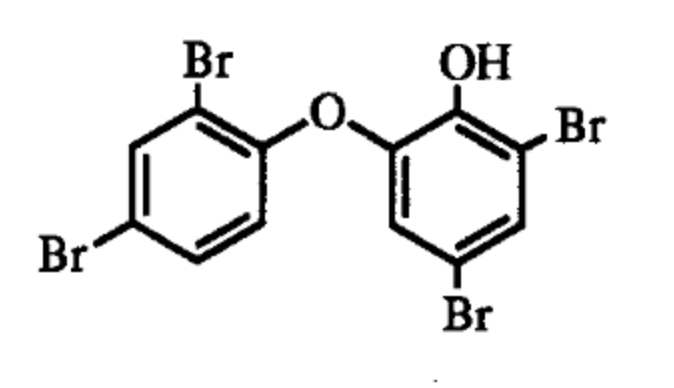 One of the Dysidea polybrominated biphenyl ethers
One of the Dysidea polybrominated biphenyl ethers
It was reported that similar compounds present in the shallow water sponge Lamellodysidea herbacea are active as chemical defense against fish feeding and environmental relevant bacteria, especially pathogenic bacteria. These properties might be one reason for the widespread occurrence of that sponge in Indonesia and the Indo-Pacific (Faisal MR et al., Mar Drugs 2021, 19(11), 611).
Cannabidiol (CBD) or 2-[(1R,6R)-6-isopropényl-3-méthylcyclohex-2-én-1-yl]-5-pentylbenzène-1,3-diol is the first phytocannabinoid discovered in 1940 in hemp (Cannabis sativa). It is one of more than 110 similar compounds (cannabinoids) in that plant. Despite numerous clinical studies, CBD is used as dietary supplement but with unproven claims of precise therapeutic effects (Wikipedia).
The lack of fundamental knowledge about some scientific aspects of CBD and of parent molecules stems from a worldwide prohibition (Duggan PJ, Aust J Chem 2021, 74, 369).
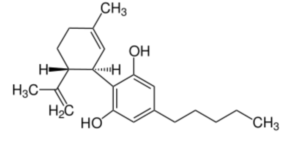 Cannabidiol (CBD)
Cannabidiol (CBD)
Tetrahydrocannabinol (THC) is the principal psychoactive constituent of cannabis. Despite the presence of multiple isomers, the term THC refers to the Delta-9-THC isomer with the chemical name (−)-trans-Δ9-tetrahydrocannabinol, a molecule related to terpenes which may be considered as a CBD derivative. Investigations have demonstrated that, when smoked, THC is absorbed into the bloodstream and reaches the brain, combining with endocannabinoid receptors located in the cerebral cortex, cerebellum, and basal ganglia, parts of the brain responsible for thinking, memory, pleasure, coordination and movement.
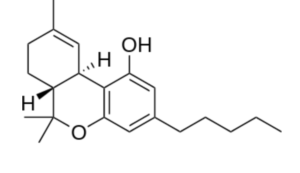 Tetrahydrocannabinol (THC)
Tetrahydrocannabinol (THC)
During investigation of natural products in marine microorganisms, 2,4,6-triphenyl-1-hexene was isolated from a marine-derived Bacillus sp. strain called APmarine135 (Kim HY et al., Mar Drugs 2024, 22(2), 72). In vitro investigations have suggested that this component is a promising anti-melanogenic agent in the cosmetic industry. This lipid has been previously isolated from a fungus Phellinus pini and a marine bacteria Solwaraspora sp. It has been also determined that it exhibited estrogenic activity in MCF-7 cells.
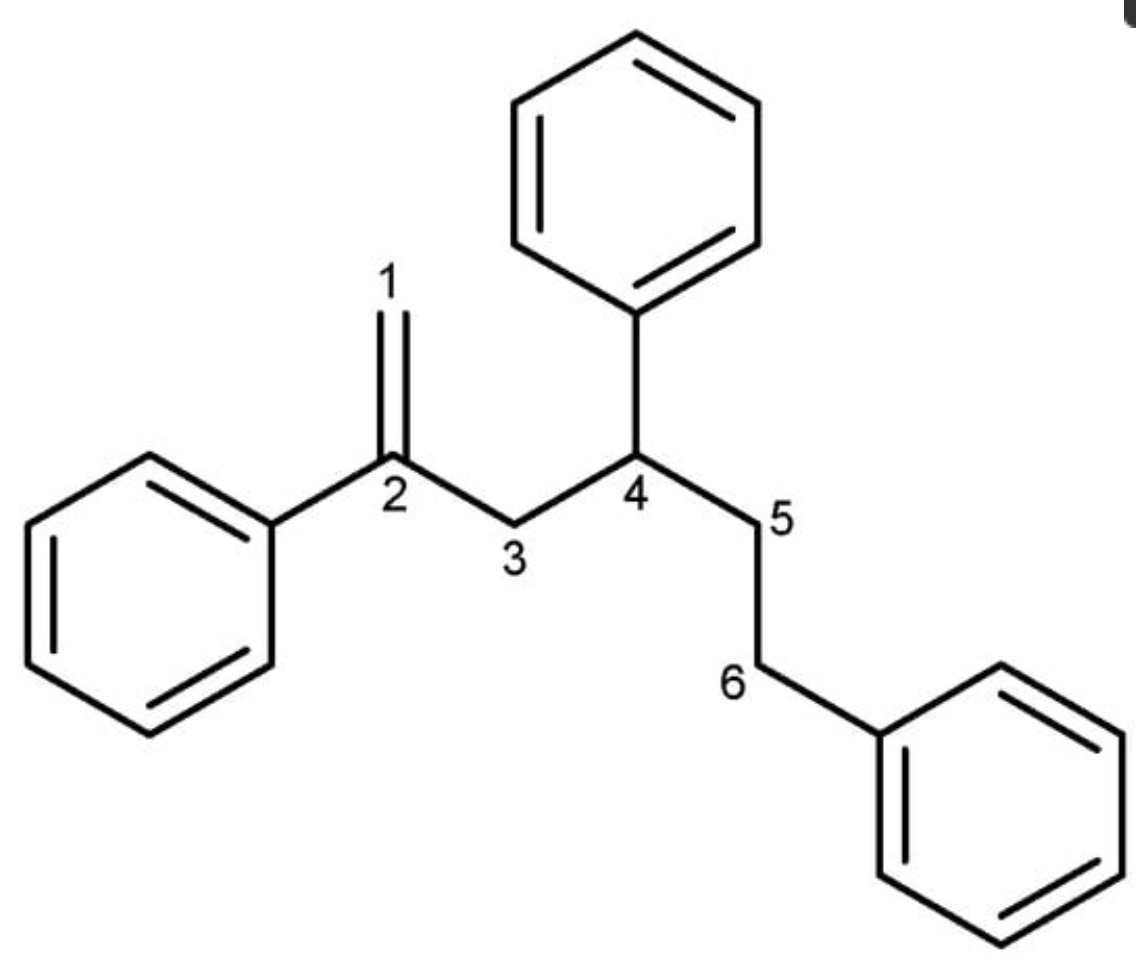
2,4,6-Triphenyl-1-hexene
Clemastine (also known as meclastin) is an oral antihistamine that works by promoting the development of myelin-making cells, called oligodendrocytes (Wikipedia). An important trialled to considered that Clemastine administration may be a remyelinating therapy for multiple sclerosis (Green AJ et al., The Lancet 2017, 390, 10111, 2481) but clinical trial was halted in early 2024 due to important side effects in some patients.
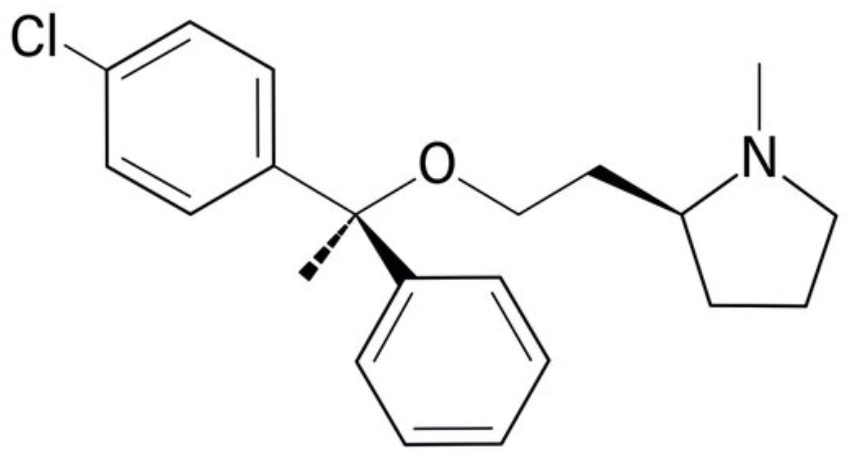 Clemastine
Clemastine
These hydrocarbons, whose only few groups are present in plants, contain fused rings containing only carbon (PAH) or have heterocycles including foreign atoms such as oxygen or nitrogen.
– Polycyclic aromatic hydrocarbons containing only carbon (PAH) :
PAH consist of two or more fused aromatic rings: PAH with less than five aromatic rings are called light PAH, when five and more rings are present they are called heavy PAH (HAP). Most of the HAP are genotoxic, 8 of them being classified as a carcinogen of category 2. EC regulation has decided a limit of 2 μg/kg, and 10 μg/kg limit for the sum of 4 PAH.
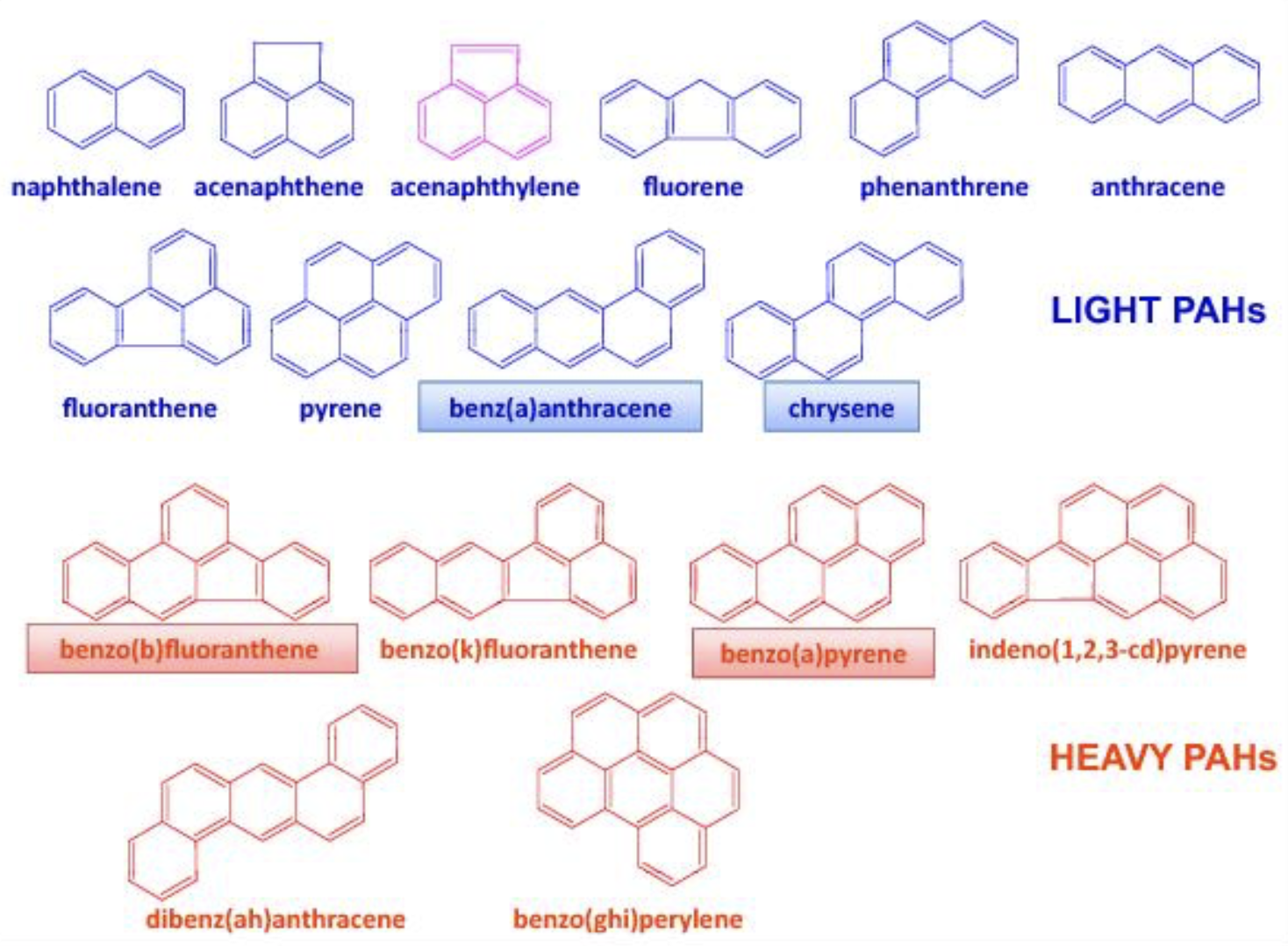
Structure of PAH accoding to Lacoste F (OCL 2014, 21, A103)
The great majjority of PAH found in living matters are formed by incomplete combustion or pyrolysis of organic material such as wood, petroleum products, coal or food. Thus, they are detected in different types of food particularly grilled meat, cereals, fats and oils. It has been several times demonstrated that barbecued foods produce carcinogenic polycyclic aromatic hydrocarbons such as benzo[a]anthracene, chrysene, benzo[b]fluoranthene, and benzo[a]pyrene due to incomplete charcoal combustion, direct food-fuel contact, and excessive heating (Wong S et al., J Food Comp Anal 2025,
137, Part A, 106890).
Their presence in vegetable oils (mainly coconut oil, pomace olive oil and sunflower oil) may result from the production process or environmental contamination. Light PAH are eliminated by the deodorization step while heavy ones are eliminated during the bleaching step.In insects.
Naphthalene is a constituent of Magnolia flowers (Azuma H et al., Phytochemistry 1996, 42, 999). It may function as protection of tissue against chewing insects and it may attract insects to pollinate by the UV absorption of accumulated naphthalene in the floral parts and floral scent. Naphthalene, playing a role of insect repellent, was shown to be produced by Muscodor vitigenus, a novel endophytic fungus (Daisy BH et al., Microbiology 2002, 148, 3737).
In insects, it has been found that naphthalene is present in extracts of the nest of Formosan subterranean termites. This is the first time naphthalene has been found naturally associated with any insect species (Chen J et al., Nature 1998, 392, 558).
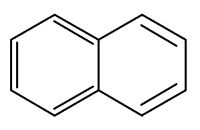
Naphthalene
Polychlorinated naphthalene (PCN) are produced upon treatment of naphthalene with chlorine, they are a group of synthetic industrial chemicals with high thermal stability and inertness. Commercial PCNs are mixtures of up to 75 components and byproducts. PCNs were once used in insulating coatings for electrical wires, as well as other applications. PCNs are also unintentionally produced and released during high-temperature industrial processes, including waste incineration, cement production, and non-ferrous smelting. While some PCNs can be broken down by sunlight and by certain microorganisms, many PCNs persist in the environment.
Perylene is a typical polyaromatic hydrocarbon that occurs in many sediments. Possible precursors include binaphthyl compounds derived from fungi, as those cited above, in which case a high abundance of sedimentary perylene might indicate a moist and humid continental climate in the depositional environment (Suzuki N et al., Org Geochem 2010, 41, 234). Perylene has the chemical formula C20H12 and occurrs as a brown solid. It or its derivatives may be carcinogenic, and considered as a dangerous pollutant. Perylene is used as a fluorescent lipid probe for cell membrane cytochemistry.
Perylene
Several characteristic larger polycyclic aromatic hydrocarbons (heavy PAH) such as the eight-ring 1,2,3,4,5,6-hexahydrophenanthro[1,10,9,8-opqra]perylene (HHPP) have been isolated from fossil crinoids which contain phenanthroperylene quinone pigments. The diagenetic origin of these molecules has been studied in fossil crinoids (Wolkenstein K, Org Geochem 2019, 136:103892).
 HHPP
HHPP
Diamondoids : they are variants of the carbon cage molecule known as adamantane (C10H16), the smallest unit cage structure of the diamond crystal lattice.
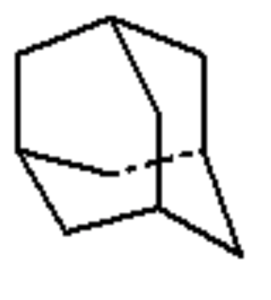 Adamantane
Adamantane
They may include one or more cages (adamantane, diamantane, triamantane, and higher polymantanes).
Diamondoids occur naturally in petroleum deposits and have been extracted and purified into large pure crystals of polymantane molecules having more than a dozen adamantane cages per molecule. It has been shown that the diamondoids found in petroleum (crude oil and condensates), coal and sedimentary rock in the geosphere and are considered to form during early diagenesis. They are composed of carbon from biological sources. These compounds are widely used to reflect thermal maturation of high mature source rocks or oils and oil cracking extents.
High concentrations of sulfated diamondoids (thiadiamondoids) together with elevated H2S are indicative of the occurrence and extent of thermochemical sulfate reduction.
Propellanes : Propellanes are polycyclic compounds in which three ring systems share a single bond. They form an exciting class of tricycloalkanes that covers a wide array of natural and synthetic products. The most studied natural product containing a propellane core is modhephene. This sesquiterpene was isolated in 1978 from Isocoma wrightii (Asteraceae) (Zalkow LH et al., J Chem Soc, Chem Commun 1978, 420). Since then, it was found in different plants of the Asteraceae family, such as in the roots of Silphium perfoliatum, in the rhizomes of Echinops giganteus and in many species of the Berkheya genus.
 Modhaphene
Modhaphene
Several other natural products that have a [3.3.3]propellane core including modhephene derivatives were isolated from different plant sources. A review of the occurrence, synthesis and applications of natural and designed [3.3.3]propellanes may be consulted (Dilmaç AM et al., Nat Prod Rep 2020, 37, 224).
1-Naphthaleneacetic acid is a synthetic plant hormone in the auxin family and is an ingredient in many commercial horticultural products. it is a rooting agent and used for the vegetative propagation of plants from stem and leaf cuttings. It is also used for plant tissue culture (Wikipedia).
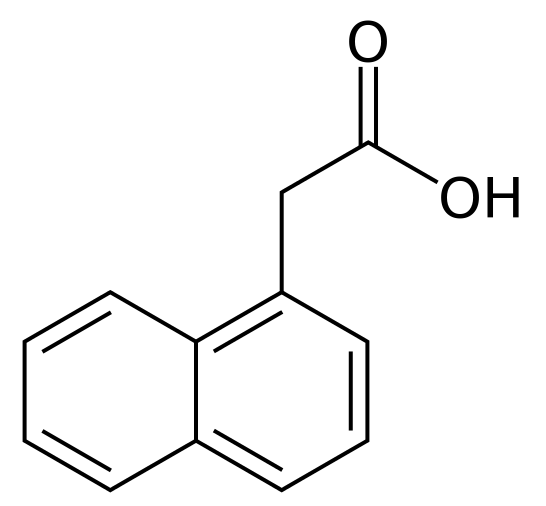 1-Naphthaleneacetic acid
1-Naphthaleneacetic acid
Naphthoquinones are present in the secretion of scent glands of Opiliones (Raspotnig G et al., J Chem Ecol 2010, 36, 158). The two main components which serve chemical defense in these animals are 1,4-naphthoquinone and its methylated derivative 6-methyl-1,4-naphthoquinone.
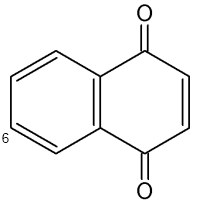
1,4-Naphthoquinone
Derivatives of 1,4-naphthoquinone were found in sea urchin coelomocytes (red spherule cells), mainly echinochrome A (or spinochrome A), (7(2)-ethyl-2, 3, 5, 6, 8-pentahydroxy 1, 4-naphthoquinone, known as one polyhydroxynaphthoquinone) which is located within cytoplasmic vesicles. Il can also be isolated from the urchin spines. Despite their identification over a century ago, and evidence of antiseptic properties, some progress has been made in characterising the immunocompetence properties of that product. It is the most studied molecule of this family and is an active principle approved to be used in humans, usually for cardiopathies and glaucoma. It is used as the active ingredient of the commercially available drug Histochrome® (Mishchenko NP.et al., Pharm Chem J 2003, 37, 48). as a cardioprotective and antioxidant drug. It is also used in ophthalmology for the treatment of macular degeneration, cornea and retina degenerative diseases. It was found that co-treatment with echinochrome A is able to prevent this decrease in membrane potential and increase in ROS level (Jeong SH et al., Marine Drugs 2014, 12, 2922).
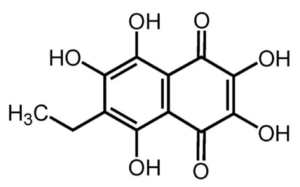 Echinochrome A
Echinochrome A
Most importantly, it has been shown that echinochrome A contributes to the modulation of the immune system. It can regulate the generation of regulatory T cells and inhibit pro-inflammatory cytokine production, These mechanisms suggest that it could be a candidate drug to alleviate the cytokine storm syndrome (review in: Rubilar T et al., Mar Drugs 2021, 19, 267). Echinochrome is clinically used in cardiology and ophthalmology based on the unique properties to block various links of free radical reactions. Numerous studies have demonstrated the effectiveness of that compound in various disease models concerning ophthalmic, cardiovascular, cerebrovascular, inflammatory, metabolic, and malignant diseases (Kim HK et al., Marine Drugs 2021, 19/ 8, 412).
Due to the broad applicability of the underlying mechanism, the therapeutic potential of echinochrome A for various other candidate diseases such as inflammatory bowel disease, gastric ulcer, atopic dermatitis, and cystic fibrosis, has been vigorously investigated. It promotes the maintenance and regeneration of the intestinal epithelium, suggesting possible beneficial effects on the intestine when used as an oral medication (Ahn JS et al., Mar Drugs 2022, 20, 715). Given their biological properties, the extraction of polyhydroxynaphthoquinones for potential medical applications has received increasing attention (Roncoroni M et al., Mar Drugs 2024, 22(4), 163).
Rhinacanthin C is a naphthoquinone isolated from Rhinacanthus nasutus (an Acanthaceae plant native to tropical Asia and the western Indian Ocean) and has been shown to exhibit antiviral activity. It has a role as a metabolite, an antiviral agent and an antineoplastic agent. It is a carboxylic ester, an enol, an enoate ester and a hydroxy-1,4-naphthoquinone. It could have a future important place in the therapy of Parkinson disease since it has been shown to significantly restored the dopamine, norepinephrine and serotonin level in brain tissue of treated mice (Saleem U et al., J Food Biochem 2021, 45/4, e13677).
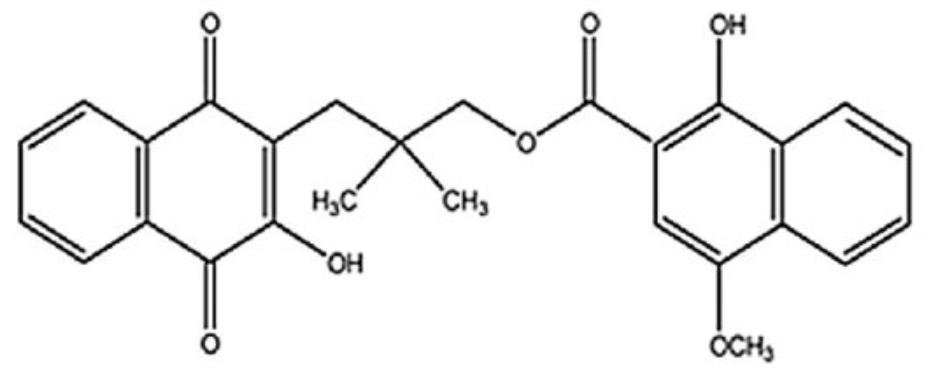 Rhinacanthin C
Rhinacanthin C
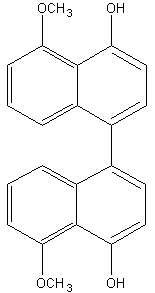
Binaphthyl compounds have been isolated from the fungus (Ascomycetes) Daldinia concentrica (Hashimoto T et al., Chem Pharm Bull 1994, 42, 1528). One of them is shown below.
Phenanthrenes : several forms of phenanthrenes are present in in higher plants, mainly in Orchidaceae family but also in Dioscoreaceae, Combretaceae, Euphorbiaceae, Juncaceae and Hepaticae. The original phenanthrene molecule may be substituted in various positions by hydroxyl, methoxyl, methyl and/or prenyl groups. Most of them are monomeric but some dimeric and trimeric forms were described. As an example, the compound below (denthyrsinin) is reported in the orchid species Cymbidium pendulum, Dendrobium spp, Eulophia nuda, Nidema boothii, Scaphyglottis livida, Thunia alba. That compound, as others, displayed potent cytotoxic activities (review in Kovacs et al., Phytochemistry 2008, 69, 1084). Many plants producing phenanthrenes are used in traditional medicine, likely in connection with the cytotoxicity, anti-microbial, spasmolytic, anti-allergic, anti-inflammatory activities of the natural phenanthrenes present in these plants.
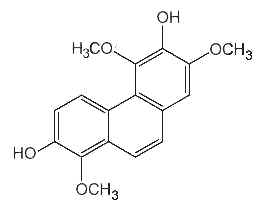
Denthyrsinin
New 9,10-dihydrophenanthrenes and phenanthrenes were isolated from Juncus setchuensis, a plant which has long been regarded as an antipyretic and detumescence agent in traditional Chinese medicine (Wang XY et al., J Nat Prod 2009, 72, 1209). Some of these compounds have shown strong antitumor and antialgal activities.
6,7-Dihydroxy- 2,4-dimethoxyphenanthrene has been shown to be the key bioactive compound from Chinese yam (Dioscorea opppsita) which has been widely used in invigorating intestines and improving long-term diarrhea according to the records of Chinese Pharmacopoeia (Li Q et al., Food Chem 4 October 2024, 141534). Furthermore, it has the strongest anti-inflammatory activity than other phenanthrenes.
Investigations have revealed that this compound was mainly digested and metabolized in the intestine, mainly into 2,6-dimethoxy-4-phenanthrenol and 1-methoxyphenanthrene which have the highest COX-2 enzyme inhibitory activity (Li Q et al., Food Chem 2025, 464, 141534).
 6,7-Dihydroxy- 2,4-dimethoxyphenanthrene
6,7-Dihydroxy- 2,4-dimethoxyphenanthrene
Hydroxylated derivatives of phenanthrene (dryhydroeffusol and effusol), isolated from Juncus effusus have anxiolytic and sedative effects, which could be used as novel anxiolytic natural products derived from herbal medicine (Singhuber J et al., Planta Med 2012, 78, 455).
Aristolochic acid 1 (8-methoxy-6-nitro-2H-phenanthro[3,4-d][1,3]dioxole-5-carboxylic acid) is a very potent and dangerous compound with a phenanthrene core. It is commonly found in the flowering plant family Aristolochiaceae and has carcinogenic, mutagenic, and nephrotoxic properties (Wikipedia).
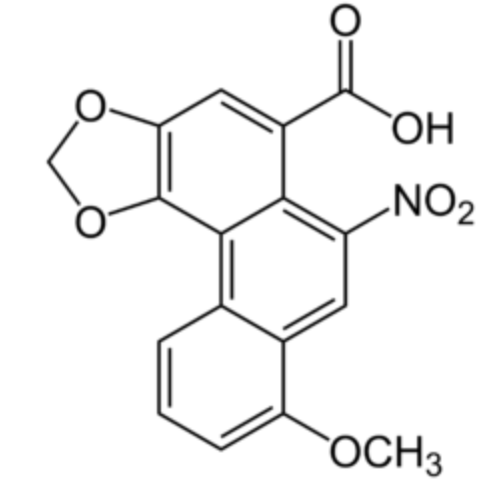
Aristolochic acid 1
Anthraquinones : the anthracene nucleus is present in several compounds detected in plants used in traditional medicine. Thus, anthraquinone is found in several plant species (Aloes), fungi and lichens but also in insects where they play a role of pheromone.
Studies on the phytochemical composition of different species of rhubarb, Rheum genus (Polygonaceae), were undertaken as early as at the beginning of the 20th century, and provided information on the presence of a variety of anthraquinone (review in Kolodziejczyk-Czepas J et al., Phytochem Rev 2021, 20, 589). The chemistry and biological activities of anthraquinones and their analogues from marine-derived fungi has been reviewed (Ghoran SH et al., Marine Drugs 2022, 20(8), 474).
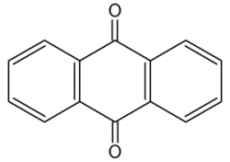
Anthraquinone
Aspergillus cristatus, a probiotic fungus isolated from Fuzhuan brick tea, produces various valuable anthraquinone metabolites metabolized which possess promising bioactivities and influence the tea fermentation process. Twelve anthraquinones were identified as (+)-variecolorquinone A, fallacinol, (+)1-O-demethylvariecolorquinone A, dermolutein, citreorosein, endocrocin, questin, rubrocristin, emodin, catenarin, physcion and erythroglaucin (Xie Z et al., Food Chem 30 January 2025, 143104). Functionally, these compounds demonstrated antioxidant, anti-inflammatory and antibacterial effects and hold promise as stable colorants and effective preservatives in food industry.
Hydroxyanthracene derivatives are a class of chemical substances naturally occurring in different botanical species and used in food to improve bowel function. EFSA has released advice concluding that hydroxyanthracene derivatives should be considered as genotoxic and carcinogenic unless there are specific data to the contrary. The hydroxyanthracene derivatives considered relevant for this risk assessment were those found in the root and rhizome of Rheum palmatum and/or Rheum officinale and/or their hybrids; leaves or fruits of Cassia senna and/or Cassia angustifolia; bark of Rhamnus frangula, bark of Rhamnus purshianus and in leaves of Aloe barbadensis and/or various Aloes pecies, mainly Aloe ferox and its hybrids.
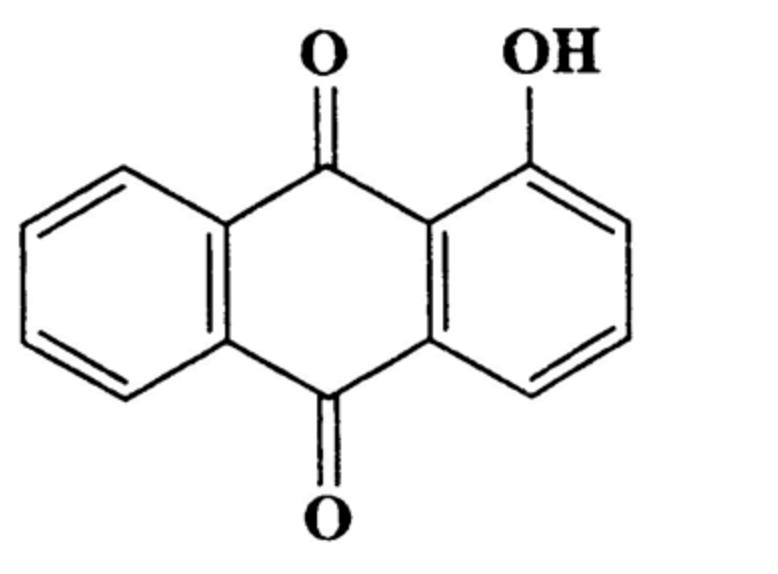 Hydroxy anthracene
Hydroxy anthracene
Among the various derivatives, emodin (6-methyl-1,3,8-trihydroxyanthraquinone) is the most important. It is an active component of several plants used in traditional Chinese medicine which have laxative, antibacterial and antiinflammatory effects. On the basis of information, the European Food Safety Authority has concluded that hydroxyanthracene derivatives in food (i.e. Aloe extracts) can improve bowel function, but advised against long-term use and consumption at high doses due to potential safety concerns such as the danger of electrolyte imbalance, impaired function of the intestine and dependence on laxatives.
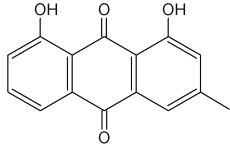
Chrysophanol is a member of the anthraquinone family. Pharmaceutical studies have shown that it exerts a number of biological effects, including anticancer and antimicrobial. The mechanism underlying the anti-inflammatory effects of chrysophanol is likely through the inhibition of caspase-1(Kim SJ et al., Molecules 2010, 15, 6436).
Chrysophanol
The anticancer potential of anthraquinones derived from marine sources and their genotoxic and mutagenic potential have been described in a review (Greco G et al., Mar Drugs 2021, 19, 272).
Several anthraquinones are considered as bioactive constituents in Cassiae seeds (Chunjuan Y et al., J Ethnopharmacol. 2015, 169, 305). The species the most studied, Cassia obtusifolia belongs to Leguminosae and known as ‘Jue Ming-Zi’ in China, which has been used in adjuvant therapy for various diseases such as hyperlipidemia, diabetes, Alzheimer’s disease, acute liver injury, inflammation, photo-phobia, headache, dizziness and hypertension.
One of these anthraquinones, obtusin, was shown to be a strong inhibitor of human thrombin, thus being a potential therapeutic strategy for the prevention and treatment of cardiovascular and thrombotic diseases.
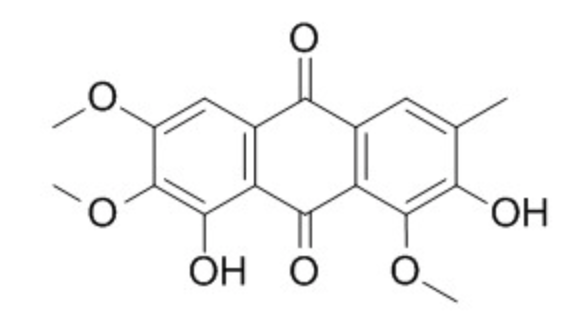
Obtusin from Cassiae seeds
Emodin (6-methyl-1,3,8-trihydroxyanthraquinone) can be isolated from rhubarb, buckthorn, and Japanese knotweed (Reynoutria japonica). It is specifically isolated from Rheum Palmatum. It has potent anti-inflammatory property as it inhibit lipoxygenase activities (Sharanya CS et al., Prostagl Other Lipid Mediators 2020, 150, 106453).
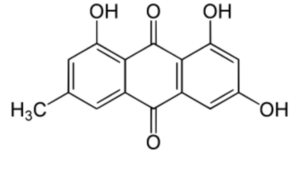
Emodin
Although not included in the living world but obviously derived by combustion of plants, many polycyclic aromatic hydrocarbons (PAHs), such as methylphenanthrene (3 cycles), triphenylene and chrysene (4 cycles), benzopyrene (5 cycles) and the coronene (6 cycles) have been identified in sediments dating from the early Triassic period to more recent times. Retene (1-methyl-7-isopropyl phenanthrene) derives here from the diagenesis of compounds which were abundantly produced by the early Palaeozoic bryophytes in the upper Silurian-lower Devonian period (Romero-Sarmiento MF et al., Org Geochem 2010, 41, 302).
Some have a structure of terpenes, they are discussed in another chapter. Many others are outside the scope of this work because they obviously come from contamination by petroleum products or their derivatives.
Several anthraquinones were isolated from Asteromyces cruciatus KMM4696 fungus associated with the brown alga Sargassum pallidum (Zhuravleva OI et al., Mar Drugs 2023, 21, 431). Several of them show a significant antimicrobial effects against Staphylococcus aureus growth. One of them, rubrumol, was among the most potent inhibitors.
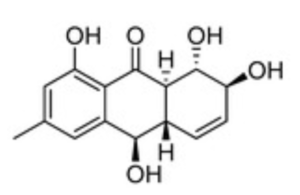 Rubrumol
Rubrumol
– Polycyclic hydrocarbons with heterocycles including oxygen, nitrogen, or several other atoms.
Xanthones : xanthones are a class of oxygen-heterocycles containing a heterocycle containing a dibenzo γ-pyrone moiety. Xanthone derivatives (sometimes referred to as xanthonoids) show a wide variety of biological activities depending on the nature and position of substituents.
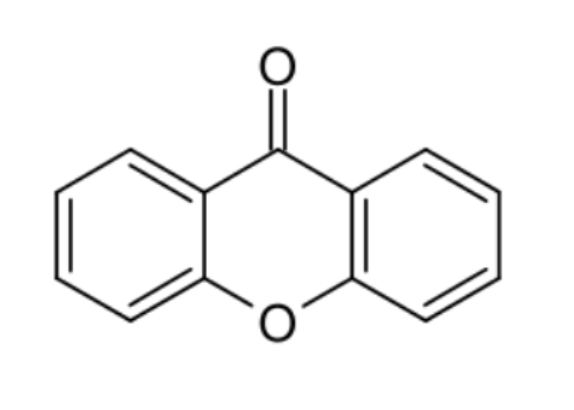 Xanthone
Xanthone
Over 200 natural xanthones have been identified, they are sometimes referred to as xanthonoids. Several are the result of a link between xanthone (or a hydroxylated derivative) and a carbohydrate (these glucosylxanthones are no longer “lipids”), for example mangiferin which has been isolated from the leaves, fruit peels, kernels and bark of Mangifera indica (the mango tree). A Mangifera indica extract (Stadice®) rich in mangiferin was said to enhance cognitive abilities in healthy adults (Jeyakodi S et al., Cureus 16(7): e65751).
Many are found in plants in the families Bonnetiaceae, Clusiaceae, and Podostemaceae, in some species of the genus Iris, and in the pericarp of the mangosteen fruit (Garcinia mangostana). Xanthone is used as an insecticide and it currently as ovicide for some moth eggs and as a larvicide. A great number of bioactive marine xanthone derivatives were reviewed with the description of their structures, biological activities, and marine sources (Soares JX et al., Marine Drugs 2022, 20, 58). Isolation, bioactivities and total synthesis of marine-derived xanthines have been reviewed (Veríssimo ACS et al., Marine Drugs 2022, 20, 347).
Sterigmatocystin is a mycotoxin structurally closely related to the aflatoxins. It contains a xanthone nucleus attached to a bifuran structure (Wikipedia). It has been found in a wide range of food products, with varying levels of contamination observed in rice, wheat, and soybeans. This ubiquitous mycotoxin is synthesized by over 50 fungal genera including Aspergillus, Emericella, Bipolaris, Botryotrichum, Humicola, and Penicillium. It is considered as a potent carcinogen, mutagen, and teratogenIt but it could be a risk to consumers only in special or unusual circumstances (Zhoo Y et al., Food Chem 2025, 19 May, 144814).
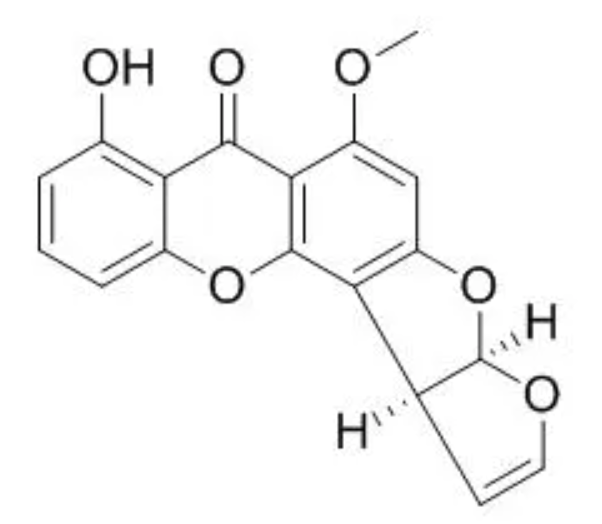 Sterigmatocystin
Sterigmatocystin
Pyran compounds : A saturated pyran ring (a tetrahydropyran), 2,4-dimethyl-5-hexanolide, was shown to be an important and specific trail pheromone in an ant species (Camponotus modoc) (Renyard A et al., J Chem Ecol 2019, 45, 901).
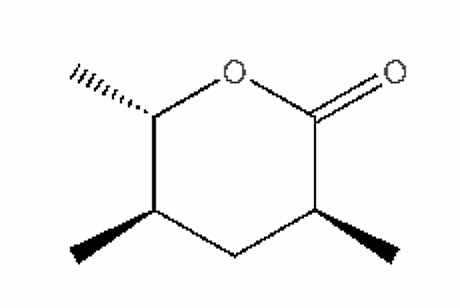
2,4-Dimethyl-5-hexanolide
Benzopyran (Chromene)- This organic and lipidic compound is bicyclic andd results from the fusion of a benzenic nucleus and a pyrane nucleus. In recent years, there have been increasing reports on the application of natural benzopyran compounds in the discovery of new pesticides, especially osthole and coumarin. A systematic and comprehensive review of these active compounds in the discovery of new agricultural chemicals was promoting the discussion and development of benzopyran active compounds (Liu X et al., J Agric Food Chem 2024, 72, 22, 12300).
Coumarins : the simplest compound of this group is coumarin (1,2-benzopyrone). There are several other derivatives by various additions. coumarins belong to the family of lactones (2H-1-benzopyran-2-one, 1,2-benzopyrone or benzo-α-pyrone) and consist of fused benzene and α-pyrone rings. A general classification considering its structure would be simple coumarins, furanocoumarins, pyranocoumarins, dihydrofurano coumarins, phenylcoumarins and bicoumarins. Coumarin belongs to the group of chemicals named chromones or chromanones, They are the derivatives of the true chromone, i.e. 1,4-benzopyrone. That compound, an isomer of coumarin, is a derivative of benzopyrane after substitution by a ketone group on the pyrene cycle.
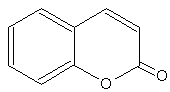
Coumarin
Coumarin is found in many plants, notably in the tonka bean from a tropical tree of the Fabaceae family (Dipteryx odorata) and cinnamon. It is produced also by the Poaceae vanilla grass (Anthoxanthum odoratum) and buffalo grass (Hierochloe odorata), and by a Rubiaceae plant, woodruff (Galium odoratum). All these plants are strongly scented due to the presence of coumarin. Coumarin is also found in many fruit such as strawberries, black currants, apricots, and cherries. As an imitation of vanilla products, it is considered as the center of compositions of the “aromatic fougere” group of scents. The originator of this group is “Fougère Royal” by the house of Houbigant, created in Paris by Paul Parquet in 1882.
Coumarin, used as rodenticide, and extracts from these plants are potential harmful as coumarin is the precursor for several anticoagulants, notably dicoumarol and warfarin.
Coumarins have been used in many research areas, such as cosmetics. It has been banned as a food additive in numerous countries since the mid-20th century. It is still used as a legal flavorant in soaps, rubber products, and the tobacco industry. However, coumarin derivatives have created a major impact in medicinal chemistry, where most of these derivatives have shown interesting pharmacological properties including anticoagulant, anti-inflammatory, antiviral, antioxidant, anticancer and inhibitory of enzymes.
Several coumarins with short- or long-chain hydrophobic groups have been described in roots of Angelica dahurica, a well-known traditional Chinese medicine (Wei W et al., Phytochemistry 2016, 123, 58), some of them having potent anti-inflammatory properties.
Umbelliferone is also known as 7-hydroxycoumarin and is a natural hydroxylated derivative of coumarin. It occurs in many familiar plants from the Apiaceae (Umbelliferae) family such as carrot, coriander and garden angelica, as well as in other plants: the mouse-ear hawkweed (Hieracium pilosella or Pilosella officinarum, Asteraceae) or the bigleaf hydrangea (Hydrangea macrophylla, Hydrangeaceae). It absorbs ultraviolet light strongly at several wavelengths. There are several indications that it is antimutagenic. It has antioxidant properties and absorbs ultraviolet light strongly but despite possible harmful mutagenic properties, is used in sunscreens.and an optical brightener for textiles.
herniarin (7-methoxycoumarin) occurs in the leaves of tha Asteraceae water hemp (Eupatorium ayapana) and in several Herniaria (Caryophyllaceae).
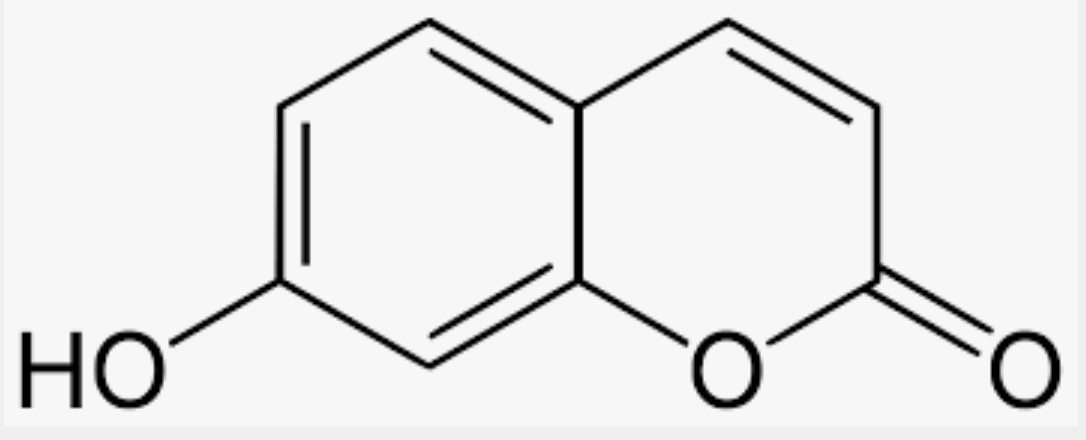 Umbelliferone
Umbelliferone
These compounds are found throughout the plant kingdom, where they give rise to numerous complex molecules. They may provide protection from ultraviolet light, against herbivores and pathogens, and mediation between plant and pollinators.
5-Methylcoumarins isolated and characterised from Clutia lanceolata, a medicinal plant native to sub-Saharan Africa and the Arabian Peninsula, strongly stimulated glucose-triggered release of insulin from β-cells (Ahmed S et al., Phytochemistry 2020, 170, 112213).
It is now well known that the secretion of coumarins is a crucial mechanism for iron uptake in nongrass species when iron availability is low, particularly in alkaline soils. Studies have identified the main coumarins involved in iron uptake and the main biochemical steps and regulatory processes controlling their biosynthesis (Robe K et al., Trends Plant Sci 2021, 26, 248)-.These findings may provide novel targets for improving plant growth and health together with iron content in the edible part of plants and thus human diet.
Several studies have reported a wide range of fusarochromanone derivatives from both terrestrial and marine fungus Fsusarium equiseti strains. The structure of fusarochromanone is unique due to the presence of two geminal methyl groups at C-2 and the alternating β-keto-amine groups. It exhibits potent antiangiogenic and antitumor activity. It has been also demonstrated that fusarochromanone exhibits significant in vitro growth inhibitory effects against glioblastomas and melanomas through induction of apoptosis and different pathways (Mahdavian E et al., BMC Res Notes 2014, 7, 601). New fusarochromanone derivatives with various biological activities are currently discovered (Pham GN et al., Mar Drugs 2024, 22(10), 444).
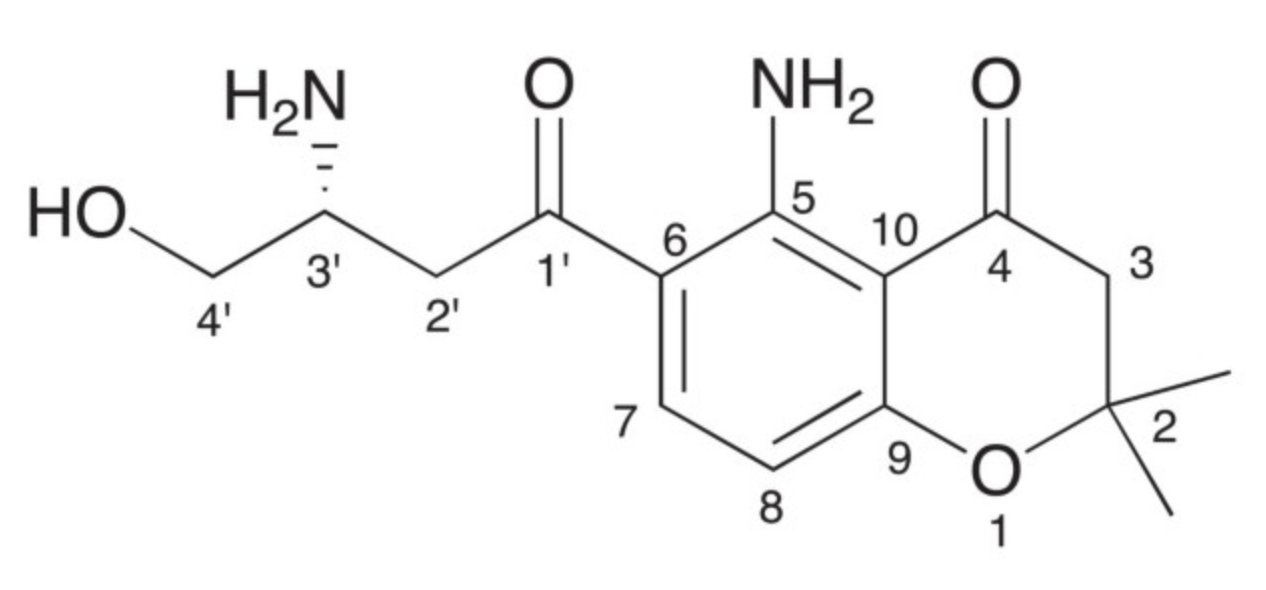 Fusarochromanone
Fusarochromanone
Some coumarin derivatives (phenylpropanoids) are present in various plants. Among them, umbelliferone, aesculetin, herniarin, psoralen and neoflavones. Scopoletin (6-methoxy-7-hydroxycoumarin) is a phytoalexin produced by several Solanaceae, such as tobacco (Nicotania tabacum), which protects plants against virus, bacteria or fungi (Oirdi ME et al., Environ Microbiol 2010, 12, 239).
The biological properties of coumarin derivatives have been studied in a variety of fields. For marine-derived coumarins, their cytotoxic properties are by far the most studied. Coumarins from oceans have been mostly found in coastal plants, bacteria, mollusks, invertebrates and sponges (Murray RD, Prog Chem Org Nat Prod. 2002, 1-619).
A review has showcased the pharmacological activities and relevant synthetic routes for specific families of derivatives and their analogues (Fernández-Peña L et al., Mar Drugs 2023, 21(1), 37). Novel methodologies has been covered to illustrate original synthetic opportunities.
Many flavonoids (flavones, flavonoid) and isoflavones have a chromone core substituted by a phenyl group in position 2 or 3. Flavones (from Latin flavus “yellow”) are a class of flavonoids based on the backbone of 2-phenyl-1-benzopyran-4-one. Flavones are common in foods, mainly from spices, and some yellow or orange fruits and vegetables. Common flavones include apigenin, naringinin, luteolin, tangeritin, and chrysin (Wikipedia). Flavonoids are the most important plant pigments for flower coloration, producing yellow or red/blue pigmentation in petals. In higher plants, they are also involved in UV filtration, symbiotic nitrogen fixation, and floral pigmentation (Wikipedia). In plants, isoflavones are found mostly (>80%) in the form of glycoconjugates and the corresponding acetyl and malonyl derivatives]. Glycosides are not readily absorbed in the gut and have only low-level estrogenic activity. Isoflavonesare are physiologically functional when these glycosides are hydrolyzed into their corresponding isoflavone-aglycones.
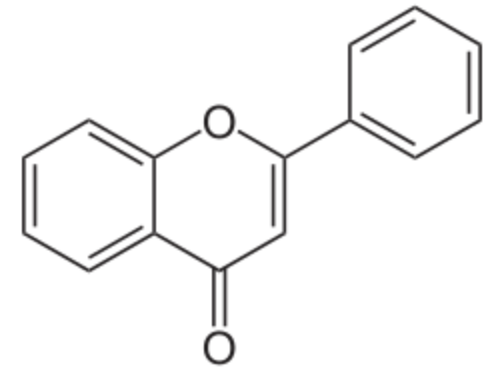 Flavone
Flavone
(2-phenyl-1-benzopyran-4-one)
Luteolin was first extracted from Reseda luteola and used as a yellow dye since the first millennium B.C. It was isolated and named, in 1829 by the French chemist Michel Eugène Chevreul. It is present in various fruits, vegetables and natural medicinal materials, including celery, broccoli, artichoke, green pepper, parsley, thyme, dandelion, perilla, chamomile tea, carrots, olive oil, peppermint, rosemary, oranges, and oregano (Wikipedia). Luteolin is known for its anti-inflammatory, antioxidant, and anticancer properties (Sung et al., J Med Food
2015, 18, 557). Cynaroside (also known as luteoloside) is a 7-O-glucoside of luteolin, it has been demonstrated to alleviate oxidative stress and cellular apoptosis across different cell lines and to inhibit cell migration and invasion in human colorectal cancer (Zhong J et al., Food Biosci January 2025, 105915).
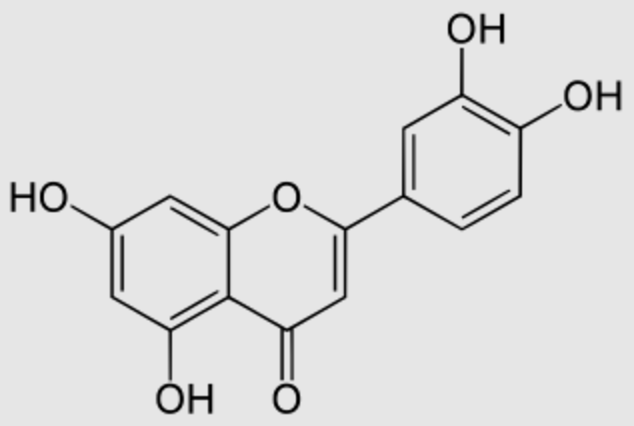 Luteolin
Luteolin
Naringenin is a flavone commonly found in citrus fruits, especially in grapefruit. High consumption of dietary naringenin is generally regarded as safe, but consuming grapefruit excessively may impair the action of anticoagulants and increase the toxicity of various prescription drugs. Naringenin may evoke CYP3A4 suppression in the liver and intestines, possibly resulting in adverse interactions with common medications (Wikipedia). It may be found both in the aglycol form, naringenin, or in its glycosidic form, naringin. Due to their capacity to fight apoptosis, inflammation, and oxidative stress, naringin and naringenin are considered valuable therapeutic agents for diabetes mellitus (Fotouhi S et al., J Function Foods 2025, 124, 106643).
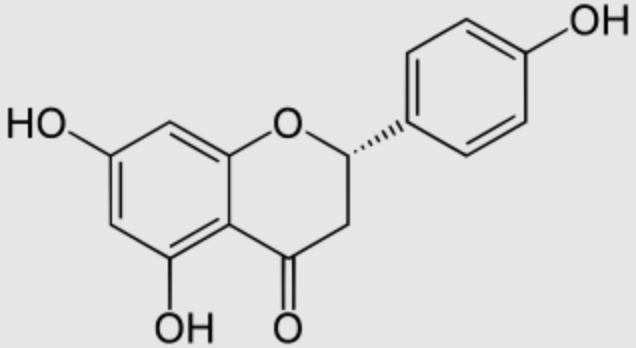 Naringenin
Naringenin
Genistein is a plant-derived, aglycone isoflavone. It has the highest content of all isoflavones in soybeans and soy products. As a type of phytoestrogen, genistein has estrogenic activity in vitro; consequently, its long-term intake by consuming soy products may affect reproductive organs, such as the uterus and breast (Wikipedia). Genistein therapy in rodent models of Altzeimer’s disease has indicated it reduces Aβ deposition and improves memory in preclinical trials (Lei H et al., J Agric Food Chem 2024, 72, 13500).
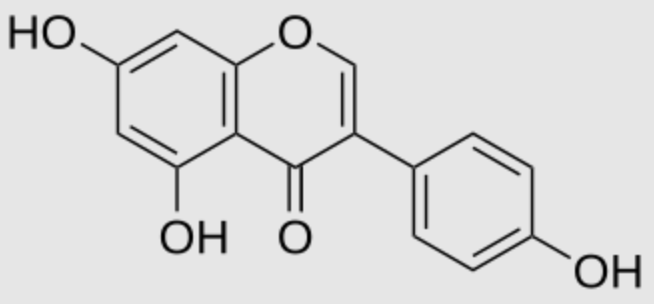 Genistein
Genistein
Equol is an agonist of estrogen receptor α (ERα) and ERβ and an active metabolite of the isoflavone daidzein. It is formed from daidzein by gut microbiota. Equol induces ERα-and ERβ-mediated gene transcription in HEC-1 cells expressing the human receptors, so human exposure to equol could have significant biological effects (Muthyala RS et al., Bioorg Med Chem 2004, 12, 1559). Analogous compounds of equol (5-hydroxy-equol, dehydroequol, 5-hydroxy-dehydroequol) are also bioactive isoflavones formed by microbial metabolism (review in: Mayo B et al., Nutrients 2019, 11, 2231).
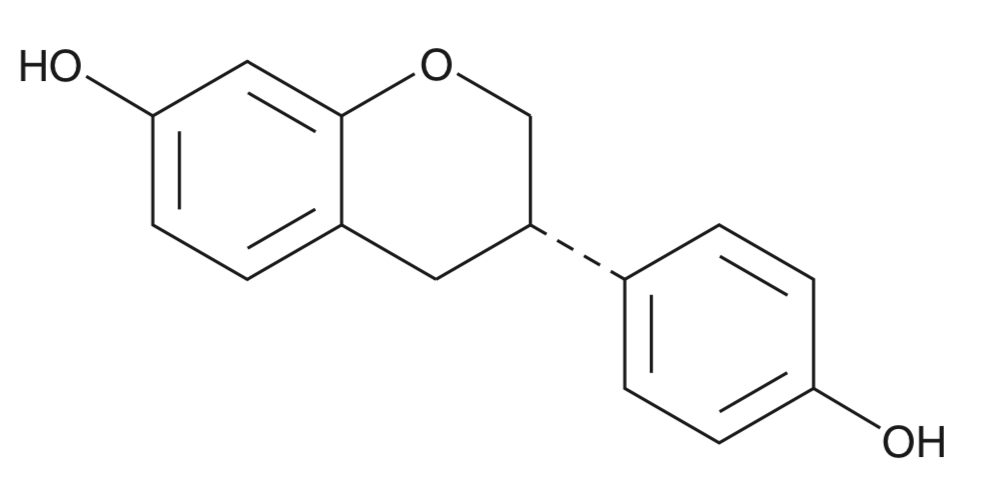 Equol
Equol
Tangeretin is a five times methoxylated flavone that is found in tangerine (Citrus tangerina) and other citrus peels. Tangeretin is commercially available as a dietary supplement. Tangeretin was also shown to have beneficial applications in other pharmaceutical, nutraceutical, and cosmetic processes. It was demonstrated to have diverse biological activities, including antioxidant, anti-inflammatory, anti-tumor, hepatoprotective, and neuroprotective effects. A study has emphasized the potentiel use of that natural chemical as an anxiolytic in mice (Husain Z et al., Food Biosci 15 November 2024, 105469). It exert anxiolytic effects in a dose-dependent manner in open-filed, hole-cross, swing, and dark-light studies in mice by elevating locomotion.
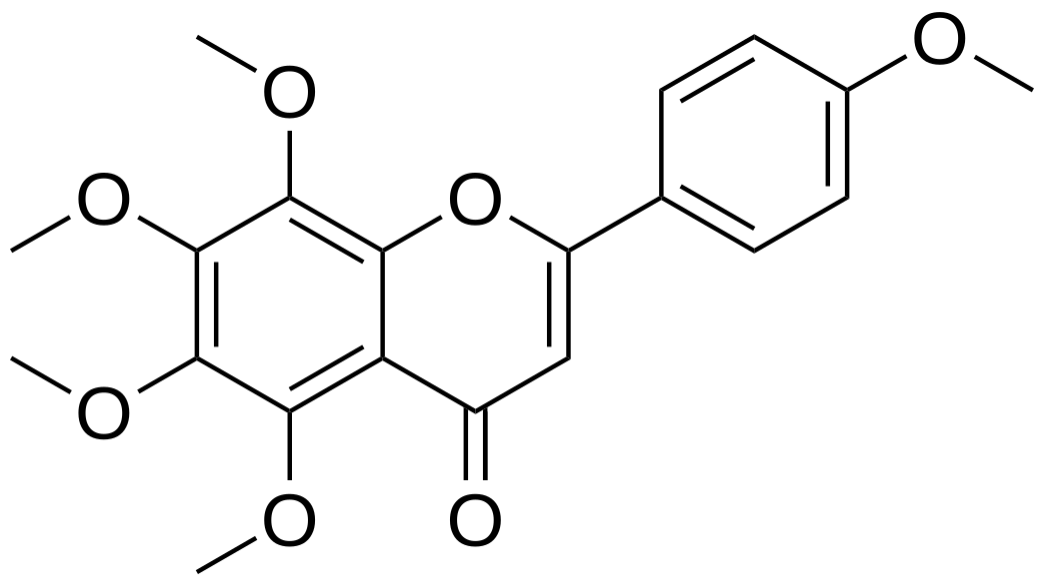 Tangeritin
Tangeritin
Nobiletin is a hexamethoxyflavone isolated from citrus peels. Its bioactive (fungicidal) capacities were demonstrated in citrus fruits, later in humans where it prevents and improves certain degenerative pathologies (Wikipedia). Several observations suggest that nobiletin has the potential to become a novel drug for the treatment and prevention of neurodegenerative diseases such as Alzheimer’s disease and Parkinson’s disease (Nakajima A et al., Int J Mol Sci 2019, 20, 3380).
Nobiletin has also fascinating anti-cancer properties. It has been shown to induce the generation of endogenous ROS by cancer cells, leading to damage to critical macromolecules and finally cell death (review in: Huang J et al., Apoptosis 2022, 27, 297).
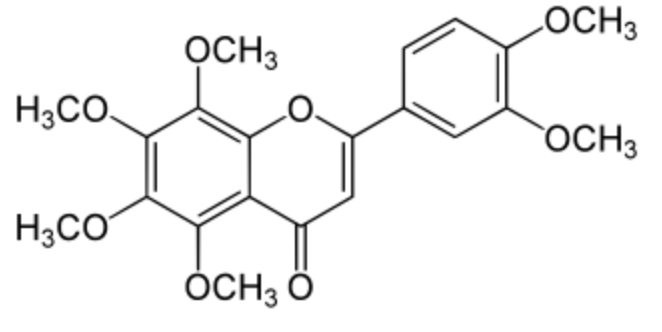 Nobiletin
Nobiletin
Hydrangenol is a flavonoid present in Hydrangea macrophylla (Hydrangeaceae), in free form or as 8-O-glucoside. It has been used to treat allergic reactions. Several studies have reported that it has also anti-inflammatory, anti-diabetic, and anti-malarial activities. A study reported on the mode of action of hydrangenol as an inhibitor of bladder cancer (Shin SS et al., EXCLI Journal 2018;17:531). It was also suggested that hydrangenol can prevent cutaneous wrinkle formation by reducing matrix metalloproteinase and inflammatory cytokine levels and increasing the expression of moisturizing factors and antioxidant genes (Myung DB et al., Nutrients 2019, 11: 2354).
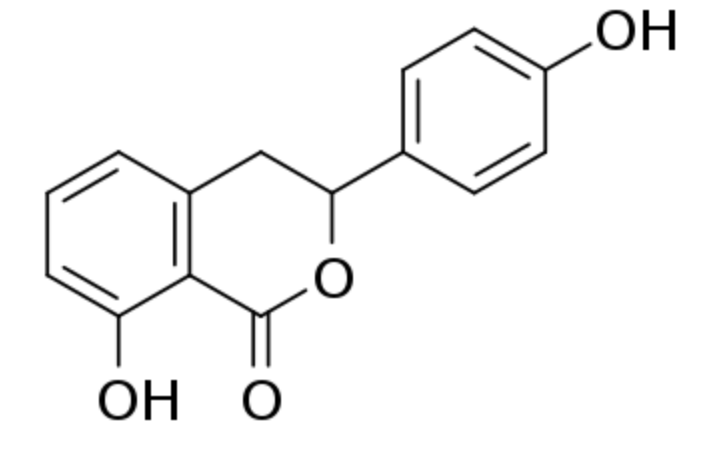
Hydrangenol
Chrysin, also called 5,7-dihydroxyflavone, is found in honey, propolis, and various plants, such as the blue passion flower (Passiflora caerulea) (Wikipedia). Chrysin is used as an ingredient in dietary supplements. Study have demonstrated that it attenuates brain aging induced by D-galactose by enhancing scavenging enzyme activities and reducing oxidative stress, neuronal apoptosis, and the impaired hippocampal neurogenesis (Prajit R et al., Biogerontology 2024 Sep 19). Ffindings underscore an important role of chrysin in inhibiting the proliferation of hepatic cancer cells and shed a light on the strategy for development the functional effects of foods (He K et al., Food Biosci 2024, 105148). The interest in anti-cancer properties of various flavonoids, including chrysin, has been reviewed (Wong SC et al., Nutrients 2023, 15, 797).
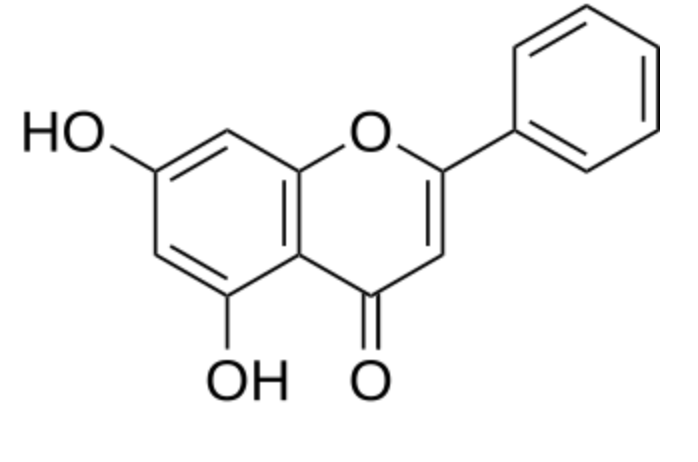 Chrysin
Chrysin
Kaempferol (3,4′,5,7-tetrahydroxyflavone) is a natural flavonol, a type of flavonoid, found in a variety of plants and plant-derived foods including kale, beans, tea, spinach, and broccoli (Wikipedia). It has attracted much attention due to its anticancer, anti-inflammatory, antioxidant, antitussive and other efficacy (Wang Q et al., Biomed Pharmacother 2023, 159,114226). Kaempferol was shown to inhibit oxidative stress and reduce macrophage pyroptosis by activating the NRF2 signaling pathway (Wang Y et al., 06 Jun 2025 PLOS ONE).
It has been observed that the kaempferol derived from Artemisia absinthium has the highest binding affinity towards the insect Helicoverpa armigera acetylcholinesterase. It has been suggested that concluded plant extracts containing that compound possess significant insecticidal properties and could be developed as botanical insecticides (Yaseen M et al., PLoS One 2025, 20, e0325959).
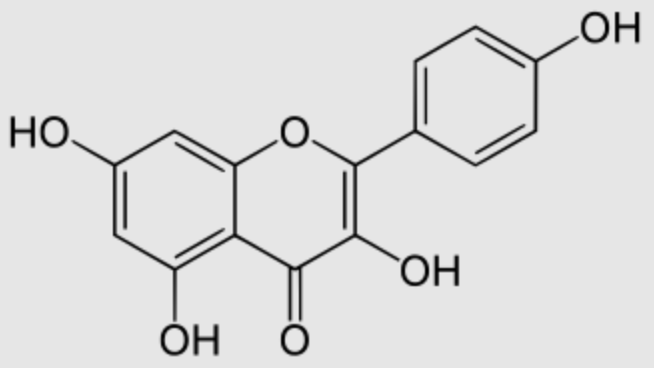 Kaempferol
Kaempferol
Auraptene is a natural bioactive monoterpene coumarin ether. It was first isolated from members of the genus Citrus. It is the geranyloxycoumarine the most abundant in nature (Fiorito S et al., Phytochem Rev 2022, 21, 317). In 1930, Nihon Kagakkai discovered its presence in sweet orange peel (Citrus sinensis). Auraptene has shown some effect as a chemopreventative agent against cancers of liver, skin, tongue, esophagus, and colon in rodent models. Studies have clearly indicated that it exerts significant effects at the central nervous system level, thus having a great potential as a neuroprotective agent.
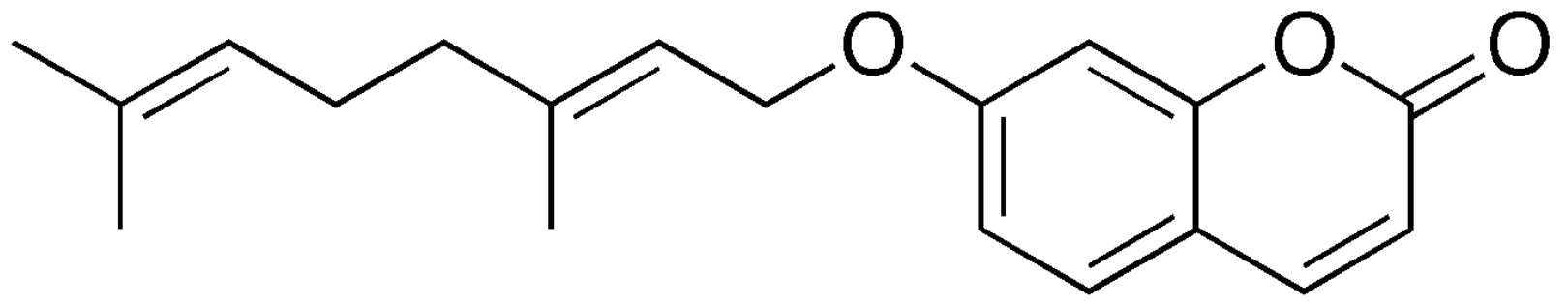 Auraptene
Auraptene
Umbelliprenin (7-farnesyloxycoumarin) has attracted the attention of several research groups due to its anti-inflammatory, immunomodulatory, and anticancer activities (Fiorito S et al., Phytochem Rev 2022, 21, 317).
 Umbelliprenin
Umbelliprenin
This oxyprenylated coumarin is commonly found in different plant species such as Ferula, Peucedanum, Seseli, Magydaris, and Ammi but also in some edible fruits and vegetables such as celery (Apium graveolens L.), wild celery (Angelica archangelica L.), and Citrus lemon. A detailed investigation on its chemical stability and its catabolism has been released (Genovese S et al., J Nat Prod 2017, 80, 2424).
Osthole (or osthol) [7-methoxy-8-(3-methylpent-2-enyl)coumarin] is a coumarin derivative found in Cnidium monnieri, a plant used in traditional Chinese medicine to treat skin affections, and in Angelica archangelica and Angelica pubescens.. Osthole was shown to exhibit several biological functions, including antiosteoporotic, antiallergic, anti-inflammatory, and antitumor functions. Recently, it was found that osthole might be a potent antidiabetic agent (Lee W.H. et al., J Agric Food Chem 2011, 59, 12874).
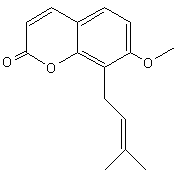
Osthole
Citrinin is a mycotoxin (a benzopyran-7-carboxylic acid derivative) which is often found in food (stored grains, sometimes fruits and other plant products). It is a secondary metabolite produced by fungi that contaminates long-stored food causing various toxic effects, like nephrotoxic, hepatotoxic and cytotoxic effects. It was first isolated from Penicillium citrinum (Wikipedia).
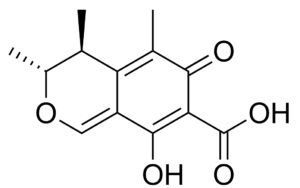
Citrinin
Citrinin was shown to have a broad antibacterial activity. It has been demonstrated that Pseudomonas aeruginosa, an opportunistic pathogen, regulates its virulence factors and biofilms through a quorum sensing system and that citrinin produced by a Penicillium species was able to inhibit the production of virulence factors, biofilm and motility by Pseudomonas (Hongrui Ji et al., Mar Drugs 2023, 21(5), 29).
– Myristicin is a phenylpropenoid compound present in small amounts in the essential oil of nutmeg and in several members of the carrot family. It is psychoactive, and acts as an anticholinergic agent. It is a precursor for the illicit synthesis of the psychedelic and empathogenic drug MMDA. Raw nutmeg contains 0.2-1.3% myristicin.
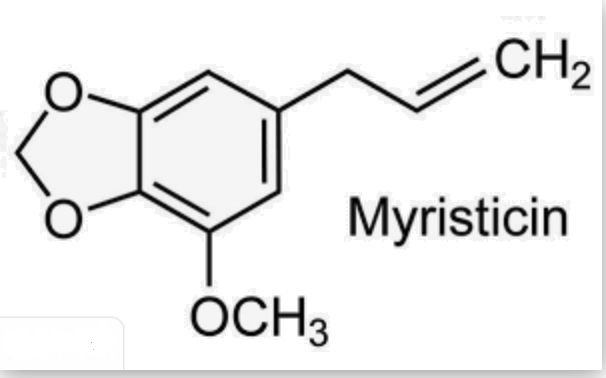
Piperine has a core structure similar to that of myristicin but with a piperidine nucleus (heterocycllic amine). It is the most active and abundant plant alkaloid in the fruit compounds responsible for the pungency of black pepper. Its amount varies from 5–10% in commercial white and black peppers. Under ultraviolet light, piperine is changed into isomers which are tasteless. Piperine was discovered in 1819 by the Danish chemist and physicist Hans Christian Ørsted, who isolated it from the fruits of Piper nigrum (Ørsted will remain famous in discovering that electric currents create magnetic fields, which was the first connection found between electricity and magnetism). The fatty acyl chains within the piperine molecule stimulate sensory nerves, activating capsaicin receptors that initiate nerve signaling, ultimately leading to the perception of pungency (review in : Zou R et al., Food Chem 2024, 456, 139980). Piperine is largely used for its effect on enhancing absorption and bioavailability of other compounds.
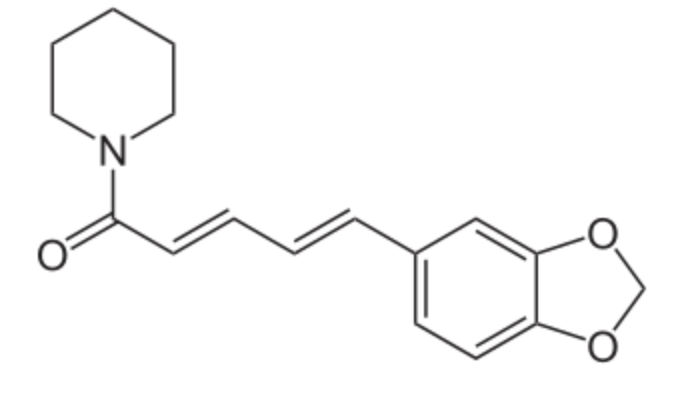
Piperine
– Psoralen (or psoralene) is a furanocoumarin. It is a derivative from umbelliferone by addition of a furan ring. Psoralen has been described in the seeds of the Fabaceae Psoralea corylifolia, but is present in many plants such as several Rutaceae (Ruta, Citrus), Moraceae, Leguminoseae Psoralea, Coronilla) and Apiaceae. Psoralen-rich plants are used in Chinese and Indian medicines and psoralene, due to its UV absorption properties, is used in treatment of psoriasis, eczema, vitiligo and in some cutaneous lymphoma. Psoralen is used in tanning accelerators, but it should be kept in mind that it increases the skin’s sensitivity to light.
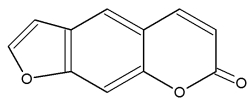
Psoralene
Bergapten is a methoxylated derivative of psoralen (on carbon 5). It is found in bergamot essential oil and in other citrus essential oils. 5-MethoxypsoralenIn was isolated in 1834 by Kalbrunner. It was the first furanocoumarin to be isolated and identified. Contact with plant parts containing bergapten (and other linear furanocoumarins) followed by exposure to ultraviolet light may lead to phytophotodermatitis. According to the International Agency for Research on Cancer, bergapten is probably carcinogenic to humans. Bergapten serves to have the skin absorb more light, and pigmentary diseases like vitiligo (leukodermia) and psoriasis in conjunction with sun exposure or solar radiation
Many furanocoumarins are toxic and are produced by plants as a defense mechanism against various types of predators and in human, some of them (bergamottin) are responsible for the “grapefruit juice effect”, in which they affect drug metabolism. Bergamottin is present in the juice but is more concentrated in the essential oil of bergamot. It is a linear furanocoumarins functionalized with a side chain derived from geraniol on the carbon 5 of the central ring. Bergamottin and dihydroxylated derivatives have been found to inhibit the human intestinal cytochrome P450 3A4 isozyme (CYP3A4) involved in the metabolism of some prescribed medications (Edwards DJ et al., Drug Metab Dispos 1996, 24, 1287). Bergamottin exhibits several characteristics that are beneficial to human health, such as anti-inflammation (Shen G et al., Front Pharmacol 2024, 15, 1389786), antioxidant, and anti-cancer effects (Santos CM et al., Fitoterapia 2023, 168, 105489).
They are also bactericide, fungicide, antiviral and insecticide and in plant they have an important role as inhibitor of germination.
 Bergamottin
Bergamottin
Pyranonaphthoquinones : they are a large group of over one hundred natural products primarily isolated from bacteria and fungi, many of which have been found to exhibit antibacterial, antifungal and anticancer properties. The basic structure of the pyranonaphthoquinones is the naphtho[2,3-c]pyran-5,10-dione ring system (see below).
Pyranonaphthoquinone natural products vary in the substitution of the pyran and aromatic rings, and can possess additional fused ring systems. A review discussed the isolation, biological activity and synthesis of pyranonaphthoquinone natural products (Naysmith BJ et al., Nat Prod Rep 2017, 34, 25), Some derivatives, named ventiloquinones, have been isolated from Ventilago harmandiana (Rhamnacea), a plant used in traditional medicine in Thailand, and have shown potent anti-inflammatory activity (Panthong K et al., Phytochemistry 2020, 169, 112182).
Butenolides are modified hydrocarbons with a hetocyclic nucleus of the furan type. The simplest one is 2-furanone.
2-Furanone
That compound is characteristic of aroma of Apiacaea (genus Angelica). It can also inhibit the quorum sensing in bacteria. Several analogues or derivatives of 2-furanone are present in fruit aroma : 4-methoxy-2,5-dimethyl-3-furanone in pineapple and 4-hydroxy-2,5-dimethyl-3-furanone (furaneol) in strawberry and tomato. Furaneol is present in roasted peanut and tobacco smoke and 3,5,5’-trimethyl-2-furanone appears in roasted in hazel-nut. Some other derivatives are active as sexual pheromones in insects.
Furaneol
Several derivatives of 2-furanone with a polyacetylenic side chain have been isolated from Asteraceae (Vernonia scorpioides) and displayed antiherpeetic activities (Pollo LAE et al., Phytochemistry 2013, 95, 375).
A review of the naturally occuring furanones may be consulted (Slaughter JC et al., Biol Rev 1999, 74, 259).
Acetogenins are polyketide products found in plants of the family Annonaceae. They have a linear 32- or 34-carbon chain containing oxygenated functional groups and terminated with a lactone or a butenolide group and one to three tetrahydrofuran rings along the hydrocarbon chain (Wikipedia).
One of the best known is Annonacin which is present in fruits of plants from the family Annonaceaea. It has toxic effects on the nervous system which could be related to atypical Parkinsonism (Wikipedia).
 Annonacin
Annonacin
Karrikins are a butenolide family of plant growth regulators found in smoke derived from burning plant material (mainly cellulose) (Flematti GR et al., Science 2004, 305, 977). These compounds are derived from 2-furanone by condensation with a pyrane group. They act as a key germination trigger for many species from fire-prone plants. It was later shown that karrikins act by a mechanism requiring gibberellic acid synthesis and light (Plant Physiol 2009, 149, 863). The most active karrikin is 3-methyl-2H-furo[2,3-c]pyran-2-one (see below). Due to the fact that it has an effect at extremely low concentrations (as low as 1 nM), it has potential as an important agronomic and horticultural chemical. A parent compound (3,4,5-trimethylfuran-2-one) has been also isolated from plant-derived smoke, but it has inversely a germination inhibiting property (Light ME et al., J Nat Prod 2010, 73, 267). The interaction of these compounds may have important ecological implications.
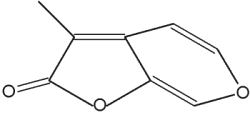
Karrikin
Brominated furanones are produced by Delisea pulchra, a marine alga endemic to the south-eastern coast of Australia. Some of which have strong inhibitory activity against fouling organisms and herbivores (de Nys R et al., Tetrahedron 1993, 49, 11213). Additionally, furanones have specific effects on colonization phenotypes of marine bacteria at concentrations found on the surface of the alga (Maximilien R et al., Aquat Microb Ecol 1998, 15, 233). Manefield M et al. have shown that these compounds inhibit acylated homoserine lactone-mediated gene expression in Escherichia coli (Manefield M et al, Microbiology 1999, 145, 283).
Extracts from the fruit of Choerospondias axillaris were shown to contain alkylated derivatives from benzofuran which displaid potent antiproliferative and cytotoxic activities against Ewing sarcoma and medulloblastoma cells, the MCT1 transporter being their target (Kil YS et al., J Nat Prod 2020, 83, 584).
 One of the most potent compounds among alkylated benzofurans
One of the most potent compounds among alkylated benzofurans
Ligustilide, a benzofuran (or phthalide) compound, is an active ingredient of umbelliferous plants such as Angelica sinensis and Ligusticum chuanxiong and is considered as the most effective biologically active ingredient in these plants widely used in traditional Chinese herbal medicine (Yang F et al., Scientific Reports 2019, 9, 6991). Ligustilide has been found to have multiple pharmacological activities, such as anti-atherosclerosis, neuroprotection, anticancer, anti-inflammatory and analgesic. Ligustilidehas been also able to significantly improved neurological function in promoting angiogenesis in vitro and in vivo (Changhong Ren et al., Neurol Res 2020 Jun 25;1-10).
Ligustilide
Dibenzofuran is an aromatic compound that has two benzene rings fused to a central furan ring. All the numbered carbon atoms have a hydrogen atom bonded to each of them. It is a volatile white solid that is soluble in nonpolar organic solvents. It is obtained from coal tar, where it exists as a 1% component.
Dibenzofuran with its derivatives Methyl-dibenzofuran and phenyl-dibenzofuran are important derivatives of dibenzofuran, and usually detected in the aromatic fraction of coal, sedimentary rocks, combustion products of municipal solid waste, and crude oil (Yang L et al., Energy Fuels 2017, 31, 3, 2513). The relative importance of their isomers may be used as maturity indicators for sedimentary rocks and crude oils.
4-Methyl dibenzofuran
4-Phenyl dibenzofuran
The core of quinolines is the 1-azanaphthalene nucleus. The simplest one is quinolin (or an isomer isoquinoline). That compound was first extracted in 1834 by a German chemist FF Runge, from coal tar. Quinoline is used to make dyes, to prepare hydroxyquinoline derivatives and niacin (nicotinic acid or vitamin B3).
Quinolin
That compound is rarely found in living material but is present, as its derivatives, in the plant Rutales and even in some insects. Quinolin is also present in some insects, as phasmids, where it plays a role against predators. Several of its derivatives are useful in diverse applications (as 8-hydroxyquinoleine).
The unique quinoline scaffold has many advantages in the discovery of new pesticides and can provide innovative and feasible solutions for the discovery of new pesticides (Cai Q et al., J. Agric Food Chem 2024, 72, 22, 12373).
Quinine is the most famous derivative of quinolin. It has been isolated in 1820 from the bark of a Cinchona tree or from Remijia. Its synthesis was accomplished in 1944 by the American chemist R.B. Woodward.
Initially, quinine is a medication used to treat malaria but this has become less common due to life-threatening side effects.
 Quinine
Quinine
A more recent derivative, hydroxychloroquine, is a medication used for the prevention and treatment of certain types of malaria, rheumatoid arthritis, and lupus.
Isoquinoline is an individual chemical specimen but is used also for the family of many thousands of natural plant alkaloids. It is a structural isomer of quinoline. Both are benzopyridines. Isoquinoline was first isolated from coal tar by Hoogewerf S et al. in 1885.
Isoquinoline
1-Benzylisoquinoline is the structural backbone found in many naturally occurring alkaloids such as papaverine.
 Papaverine
Papaverine
These alkaloids are natural products, chemically derived from isoquinoline, form the largest group among the alkaloids. They can be further classified based on their different chemical basic structures. The most common structural types are the benzylisoquinolines and the aporphines. According to current knowledge, a total of about 2500 isoquinoline alkaloids are known nowadays, which are mainly formed by plants (Wikipedia).
The bacteria Pseudomonas aeruginosa was shown to produce, besides an alkyl homoserine lactone, new cell-to-cell signal molecules (quorum sensing system). This molecule was determined to have a 4-quinolone base structure with an alkyl chain (2-heptyl-3-hydroxy-4-quinolone) and therefore has been designated as the Pseudomonas quinolone signal (Pesci E et al., Proc Natl Acad Sci 1999, 96, 11229). Similar compounds have been previously described for their antimicrobial activities (Hays EE et al., J Biol Chem 1945, 149, 725). It was found that this molecule controlled the expression of genes encoding for the major virulence factors. The maximal quinolone production occurs at the end of the exponential growth phase, supporting the hypothesis that it acts as a secondary regulatory signal for a subset of quorum sensing-controlled genes.
2-Heptyl-3-hydroxy-4-quinolone
Later, another analogue was isolated from the same bacteria, 3,4-dihydroxy-2-heptylquinoline, which could be the direct precursor of the previous quinolone and, likely, the message molecule involved in cell-to-cell communication (Deziel E et al., Proc Natl Acad Sci 2004, 101, 1339). A review of that type of quorum sensing may be read for further information (Dubern JF et al., Mol Biosyst 2008, 4, 882).
In contrast with Pseudomonas quinolone, Burkholderia spp produce several hydroxy-quinolines with a methyl group in position 3 and a hydroxyl group in position 4 and various alkyl chains (Vial L et al., J Bacteriol 2008, 190, 5339). It was later shown that these quorum-sensing signals control the bacterial synthesis of antibiotics (Duerkop BA et al., J Bacteriol 2009, 191, 3909).
A new quinoline-2-carboxylic acid, 4-hydroxy-6-methoxyquinoline-2-carboxylic acid (6-methoxy-kynurenic acid), has been isolated from Ephedra pachyclada. Kynurenic and 6-hydroxykynurenic acids, previously reported from plants, were also isolated from Ephedra (Starratt AN et al., Phytochemistry 1996, 42, 1477).
Compounds related to quinolines, the benzoxazinones, are present as inactive glucosides (phytoanticipines), mainly in Gramineae (rye, wheat, corn) (Niemeyer HM, Phytochemistry 1988, 27, 3349). They are sometimes described as cyclic hydroxamic acids. In rye, the principal compound is the glucoside of DIBOA (2,4-dihydroxy-1,4-benzoxazin-3-one), in wheat and corn, it is the glucoside of the methoxylated form, DIMBOA (2,4-dihydroxy-7-methoxy-1,4-benzoxazin-3-one).
Benzoxazinones
(R = OCH3 : DIMBOA , R = H : DIBOA)
These glucosides are hydrolyzed during the plant infection by fungi or bacteria or after insect attacks. The benzoxazinones aglycones, and their breakdown products (phenoxazinones, acetamides, and malonamic acids), behave like antifungal, antibacterial and also insecticidal substances (allelopathy). These aspects of allelopathy have been reviewed for the sustainable weed control in rye fields (Schulz M et al., J Chem Ecol 2013, 39, 154).
Investigations in genetic engineering are performed to induce the production of these protective agents in other crop plants.
Isoquinolines find many applications, including : anesthetics (dimethisoquin), antihypertension agents (quinapril), antiretroviral agents (saquinavir), and vasodilators (papaverine).
Several isoquinolines have been isolated from Zanthoxylum nitidum (or Shiny-leaf prickly-ash) and display antiproliferative effects against human cancer cell lines (Qin F et al., Phytochemistry 2023, 205, 113476).
Berberine is a molecule belonging to the group of benzylisoquinoline alkaloids found in such plants as Berberis, Mahonia, Hydrastis, Xanthorhiza, Phellodendron, Coptis, and Eschscholzia. Due to berberine’s strong yellow color, Berberis species were used to dye wool, leather, and wood. Under ultraviolet light, berberine shows a strong yellow fluorescence, so it is useful in histology and biochemistry to detect lipids on chromatograms.
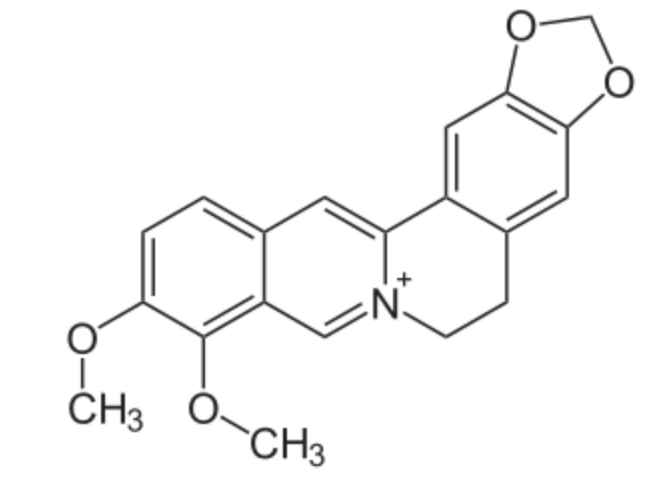
Berberin
Berberine was used in China as a folk medicine and is still a common over-the-counter medication for gastrointestinal infection in China. Studies have been conducted to determine if berberine may affect diabetes. Some research has shown that it may be used against some Staphylococcus aureus infection or diarrhea caused by enterotoxigenic E. coli. Recently, it has been reported that berberine helps in reducing cholesterol and lipid accumulations in both the plasma and in the liver (Doggrell SA, Expert Opin Investig Drugs 2005, 14, 683).
Unfortunately, there is insufficient evidence to conclude that berberine is safe or effective for any condition (Wikipedia).
Sophocarpine is one of the significant alkaloid extracted from the traditional herb medicine Sophora flavescens which has many pharmacological properties such as anti-virus, anti-tumor, anti-inflammatory. Sophocarpine inhibits the growth of gastric cancer cells inducting autophagy and apoptosis. It has been demonstrated to have also anti-tumor activity in various cancer cells, including hepatocellular carcinoma, prostate cancer and colorectal cancer. Furthermore, this molecule has a neuroprotective effect against llutamate-Induced HT22 cell cytotoxicity (Wang T et al., J Oleo Sci 2024, 73, 359).
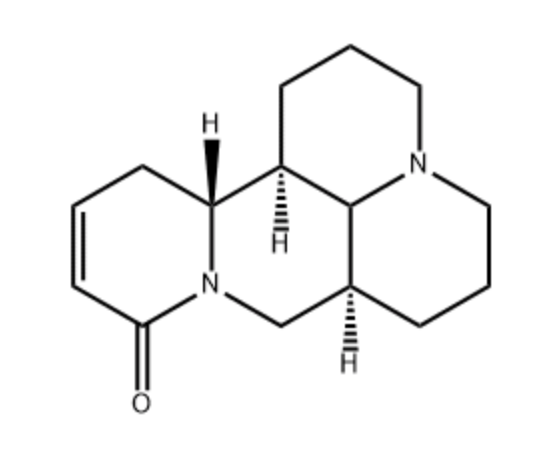 Sophorocapnine
Sophorocapnine
Methyl-Fascaplysin is one of the most powerful marine-derived compounds that produce in vitro neuroprotective effects. Fascaplysin is a extensively active benzoyl-linked β-carboline alkaloid initially extracted from the Fijian sponge Fascaplysinopsis; this molecule showed antitumoral and antioxidative effects (Naveen kumar S et al., Front Lab Med 2018, 2, 41). A study reported that fascaplysin molecule inhibits acetylcholinesterase, implying that this molecule may have positive effects on brain (Manda S et al., Eur J Med Chem 2016, 107, 1).
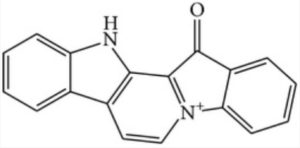
Fascaplysin
Devenez membre et participez au développement de la Lipidomique au XXIème siècle.
S'inscrire