
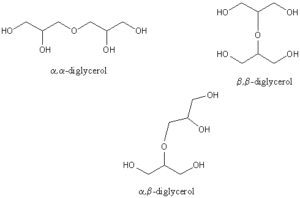
These compounds are formed chemically by esterification of fatty acids, largely saturated or mono-unsaturated, to one or several hydroxyl groups of polyglycerol (Dobson KS et al., JAOCS 1993, 70, 1089). As glycerol is a trifunctional molecule, it may condense with itself to give polymers. These polyglycerols are hydroxy-containing ethers, diglycerol being the simplest example. If the primary hydroxyls are the only ones concerned in the reaction, the products are linear, but if the secondary hydroxyl groups are also involved, branched chains are formed. Thus, several diglycerol molecules can be formed (see figure below) but if the polymerization proceeds to tri-, tetra- and higher glycerols the number of possible isomers increases exponentially ( 8 different linear isomers for a triglycerol). It has been found that cyclic products can also result from intra-molecular reactions.
Classically, 30 to 50 % of the total amount of hydroxyl groups are esterified by fatty acids. These fatty acids are formed either of one species (lauric, stearic or oleic acid) or a mixture from vegetal oils (cottonseed oil, castor oil) or from animal source (beeswax).
Chemically, polyglycerol esters may be formed by an alkaline catalyzed random polymerization of glycerol followed by an esterification with isolated fatty acids or triacylglycerols. The obtained mixture varies in polymerization degree, kind and position of esterified fatty acid (monoesters diglycerol or triglycerol or tetraglycerol, diesters diglycerol or triglycerol).
Polyglyceryl esters are important non-ionic surfactants with various applications in cosmetic, in food, pharmaceutical and other industries. Their amphiphilic character enables their use in the stabilization of various suspensions.
In cosmetics they are used to emulsify, control viscosity, disperse and stabilize the final mixture. They are incorporated into hair styling gels, skin treatment gels, skin cleansers, baby creams, long-acting hand creams, moisturizing sunscreens, and sun-protective sticks. The most powerful emulsifiers are diglyceryl diisostearate in foundation creams, diglyceryl monolaurate in makeup-removing skin cleansers, and diglyceryl monooleate in baby creams.
In foods they are used as emulsifying agents in the production of fine bakery, chewing gum, and in replacement of fats. Thus, polyglycerol polyricinoleate (E476), is an emulsifier made from castor beans (rich in ricinoleic acid) which reduces the viscosity of chocolate and similar coatings and compounds. It is used at low levels (fractions of percents). By using E476), the chocolate recipe has lower costs in terms of less cocoa butter but also gives the benefit of having less fat.
Polyglycerol esters have been touted as replacements for fats to reduce human calorie consumption. This replacement may be intended since the fatty acid esters were found to resist hydrolysis by digestive enzymes (Dobson KS et al., JAOCS 1993, 70, 1089).
DISPERSIVE LIQUID-LIQUID MICROEXTRACTION
Lire la suiteDevenez membre et participez au développement de la Lipidomique au XXIème siècle.
S'inscrire