
Among the diverse fatty acids, hydroxy fatty acids constitute a unique class, which is characterized by the presence of a hydroxyl group attached to a long aliphatic chain. The hydroxyl group(s) may occur at various positions in the carbon chain which can be saturated or monoenoic.
Some polyhydroxy fatty acids are also known, which are most frequently produced by lipoxygenase activities, as for several mono-hydroxylated fatty acids.
Fatty acid esters of hydroxy fatty acids represent a complex lipid class named estolides. They share a common chemical architecture, characterized by an “estolide bond” that links the hydroxy fatty acid backbone and the fatty acid.
In some bacteria, complex hydroxy, branched-chain fatty acids (mycolic acids) are described.
![]()
MONOHYDROXY FATTY ACIDS
α-Hydroxy acids or 2-hydroxy acids are found in plants (chain from 12 up to 24 carbon atoms) and in animal wool waxes, skin lipids and specialized tissues, mainly in brain.
A series of 2-hydroxylated fatty acid was recorded from the sea urchin Tripneustes esculentus (Carballeira NM et al., J Nat Prod 1994, 57, 619) and the sea cucumber Holothuria mexicana (Carballeira NM et al., J Nat Prod 1996, 59, 1076) and later found in abyssal holothurians (Drazen JC et al., Comp Biochem Physiol B 2008, 151, 79). Various 2-hydroxy FAs, αOH 22:0, αOH 22:1, αOH 23:0, αOH 23:1, αOH 24:0, and αOH 24:1, were identified in this sea urchin (Imbs AB et al., Comp Biochem Physiol B 2007,148, 314). Furthermore, αOH 18:0 was found in H. mexicana (Carballeira NM et al., J Nat Prod 1996, 59, 1076). However, while these are minor components (from 0.1 to 1.2% of total FAs) in sea urchins and holothurians from shallow waters, all four holothurian species from the abyssal zone contain 2-hydroxy FAs at extraordinarily high levels: 1.5–4.3% of 2-hydroxytricosenoic acid (αOH 23:1) and 10.5–14% of 2-hydroxytetracosenoic acid (αOH 24:1).
2-Hydroxylinolenic acid was reported at a level of 13% in the seed oil of the Labiateae Thymus vulgaris (Smith CR et al., Lipids 1969, 4, 9). Along with the previous one, 2-hydroxylinoleic and 2-hydroxyoleic were detected in another Labiateae Salvia nilotica (Bohannon MB et al., Lipids, 1975, 10, 703). Several 2-hydroxy fatty acids have been reported to be present in polar lipids of the alga Grateloupia turuturu from Britany, France (Kendel M et al., Lipids 2013, 48, 535).
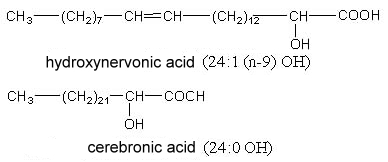
2-Hydroxytetracosanoic acid (cerebronic acid) and 2-hydroxy-15-tetracosenoic acid (hydroxynervonic acid) are constituents of the ceramide part of cerebrosides (glycosphingolipides found mainly in nervous tissue and in little amount in plants).
The testis and spermatozoa of boar and rat contain sphingomyelin with 2-hydroxylated n-6 tetra- and pentaenoic acids with very long carbon chain (up to 34 carbon atoms) (Robinson BS et al., J Biol Chem 1992, 267, 1746). It has been postulated that these lipids play a role in reproduction.
Although they comprise a relatively small class of lipids, hydroxylated fatty acids have attracted attention, due to their importance as components of animal and plant tissues, engaging in diverse biological functions (Zhang W et al., Sci. Total Environ. 2024, 906, 167358). These fatty acids are widespread in nature and are involved in biotransformation and oxidation processes in living organisms. The unique chemical and physical properties attributed to the hydroxyl group make these acids ideal biomarkers in biomedicine and environmental toxicology, as well as organic geochemistry. Furthermore, hydroxy-acids have numerous industrial applications due to their higher reactivity, viscosity, and solvent miscibility. It has been also shown that they play a crucial role in the global biogeochemical cycle as a portion of biological aerosols, which can significantly influence cloud nucleation processes and atmospheric chemistry (Bikkina P et al., ACS Earth Space Chem. 2019, 3, 366).
Several studies have uncovered potent biological activities of 2-hydroxyoleic acid (Minerval). This synthetic derivative of oleic acid was found to have potent anti-cancer activities in vitro and in animal models (Martinez J et al., Mol Pharmacol 2005, 67, 531; Llado V et al., J Cell Mol Med 2010, 14, 659). The anti-cancer activity of 2-hydroxyoleic acid is mediated, at least in part, by downregulation of dihydrofolate reductase (Llado V et al., PNAS 2009, 106, 13754). The mechanism of its action is not fully understood, and it has been attributed to its structural effects on cell membranes, rather than specific interactions with target proteins. Nevertheless, it seems that the pro-apoptotic activity of 2-hydroxyoleic acid explains the effectiveness of this non-toxic anticancer drug (Llado V et al., J Cell Mol Med 2010, 14, 659). New results have shown that this fatty acid is able to regulate the synthesis of sphingomyelin in glioma cells in restoring normal membrane levels and triggering cell cycle arrest (Barcel-Coblijn G et al., PNAS 2011, 108, 19569).
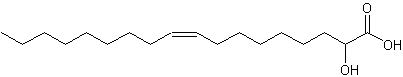
2-Hydroxy-9-cis-octadecenoic acid
The addition of 2-hydroxy palmitic acid to different cell lines increased their sensitivity to the synthetic antitumor drug, PM02734 (Herrero AB et al., Cancer Res 2008, 68, 9779).
(S)-2-Hydroxymyristic acid has been isolation from a marine bacterium, Shewanella oneidensis, and displayed inhibition for the germination of the representative soft fouling macro-algae Ulva pertusa spores and a strong antifouling activity against a wide range of micro- and macro-foulers. Furthermore, that fatty acid can be completely biodegraded, suggesting that this class of compounds can be used as environmentally friendly substitutes for toxic antifoulants (Wang KL et al., Marine Drugs 2022, 20, 90).
A review of fatty acid 2-hydroxylation in sphingolipid biology in connection with the nervous system and various cell types may be consulted (Hama H, Biochim Biophys Acta 2010, 1801, 405). Curiously, among the enantiomers, the (R)-enantiomer is enriched in hexosylceramide whereas the (S)-enantiomer is preferentially incorporated into ceramide (Guo L et al., J Lipid Res 2012, 54, 1327).
The analysis of long-chain hydroxylated fatty acids in ancient ceramics or shells enables to identify the origin of these deposits. Thus, 13,14-dihydroxydocosanoic acid and 11,12-dihydroxyeicosanoic acid, derived from erucic acid (C22:1) and gondoic acid (C20:1) were shown to be biomarkers for seed oil from Brassicaceae plants such as rapeseed (Brassica napus) or radish (Raphanus sativus) (Romanus K et al., Anal Bioanal Chem 2008, 390, 783). Goni and Hedges (1990b) showed
Leaves of angiosperms and different gymnosperm plant families could be distinguished by the abundance of 14-hydroxytetradecanoic acid and positional isomers of dihydroxyhexadecanoic acid (Goni MA et al., Geochim Cosmochim Acta 1990, 54, 3073). The hydroxylated fatty acids from leaf-derived lipids have been proposed as plant biomarkers in soils and sediments (Mueller KE et al., Org Geochem 2012, 52, 130).
Polyhydroxyalkanoates : The chemical composition of lipid inclusions in Bacillus megaterium was identifed in 1926 as poly(3-hydroxybutyric acid) (Lemoigne M, Bull Soc Chim Biol 1926, 8, 770). Since that discovery, a large variety of bacteria were shown to synthesize polyesters (polyhydroxyalkanoates) forming linear chains of esterified 3-hydroxy acids (Kim YP et al., Adv Biochem Eng Biotechnol 2001, 71, 51). More than 90 different monomer units have been identified as constituents of polyhydroxyalkanoates in various bacteria. A few members include poly(3-hydroxybutyrate) or poly(3-hydroxybutyrate-co-3-hydroxyvalerate. They are sometimes, but erroneously, considered to be a carbohydrate, but their solubility characteristics are those of a lipid, except for high molecular weight homopolymers of 3-hydroxybutyrate units which are acetone-insoluble.
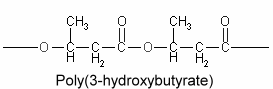
Poly(3-hydroxybutyrate) is the most widespread and best characterized lipid polymer. These polyesters accumulate as intracellular inclusions and act as a carbon and energy reserve. They are produced by some bacteria when they have extra energy, then used as an energy source when needed. They have a great industrial interest because of their plastic and elastomer properties and as a source of biodegradable polymers for low-value commodity products. Their synthesis in crop plants would allow an efficient large-scale production which may lead to new substitutes for petroleum-derived plastics (Rezzonico E et al., Phytochemistry Rev 2002, 1, 87). Genetic engineering has been done to transfer the polymer-making capacity to Escherischia coli and to higher plants. It is theoretically possible to modify starch forming plants (such as potatoes) to grow these polymers.
Several lipid-like fractions (acetone-soluble) are copolymers containing both short- and medium-chain-length 3-hydroxyalkanoate units. They have been identified as various hydroxyalkanoic acids with 3 to 14 carbon atoms (Steinbuchel A, 1991, Polyhydroxyalkanoic acids. In: Byrom D (ed) Biomaterials. Macmillan, New York, p 123). Two groups of bacteria have been described : those which produce short-chain polyhydroxyalkanoates (C3-C5 monomer units) and those producing medium-chain polyhydroxyalkanoates (C6-C14 monomer units). Some bacteria (Rhodospirillum, Rhodocyclus, Rhodococcus, Aeromonas, Pseudomonas) have been shown to accumulate polyesters containing short- and medium-chain-length 3-hydroxyalkanoic acids. A copolymer with a molecular mass of about of 40 000 containing 3-hydroxybutyric acid (3HB) and several other 3-hydroxyalkanoic acids has been described in Pseudomonas sp (Kato M et al., Appl Microbiol Biotechnol 1996, 45, 363). A part of the described structure is shown below.
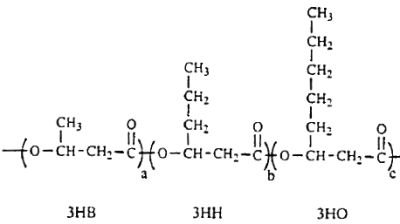
A fragment of polyhydroxyalkanoate containing 3HB (3-hydroxybutyrate), 3HH (3-hydroxyhexanoate) and 3HO (3-hydroxyoctanoate) units
Applications are being developed to produce and extract these bacterial polymers for use in industry, including molded goods, paper coatings, non-woven fabrics, adhesives, films, and polymer additives. The biocompatible nature of polyhydroxyalkanoates and their potential applications in the medical field should also not be overlooked. Biodegradable plastics and polymers are certain to increase in importance as environmental contamination and waste disposal problems associated with synthetic plastics become more severe.
Special interest has been paid to haloarchaea as they produce significant concentrations of polyhydroxyalkanoate, polyhydroxybutyrate, and polyhydroxyvalerate. The growth of those microorganisms at the pilot or industrial scale offers several advantages compared to that of other microbes producing bioplastic materials. A review summarizes the most recent findings regarding the production of bioplastics by halophilic microorganisms with special emphasis on haloarchaea (Simó-Cabrera L et al., Mar Drugs 2021, 19, 159).
The valorization of the green alga Ulva rigida was proved possible as an industrial source of poly(3-hydroxybutyrate (Leandro T et al., Mar Drugs 2023, 21, 537).
Metabolix Inc. of Cambridge, Mass USA, has taken up the challenge to further develop efficient technologies for polyhydroxyalkanoates production (http://www.metabolix.com/index.html).
β-Hydroxy acids or 3-hydroxy acids occur in some bacterial lipids. 3-Hydroxy oxylipins are widely distributed in nature, occurring in mammals, bacteria and yeasts. In mammalian systems, production of 3-hydroxy oxylipins is mainly attributed to fatty acid oxidation disorders.
Requirement for ω and (ω–1)-hydroxylations of fatty acids by human cytochromes P450 2E1 and 4A11 has been determined (Adas F et al., J Lipid Res 1990, 40, 1990).
An investigation has revealed that 3-hydroxydecanoic acid, a natural microbial-derived compound, has an effect on growth and yield of wheat (Triticum aestivum). An application at the level of 53 μM significantly enhanced seedling development, including increases in root length, fresh weight, and stem diameter. Moreover, it elevated the chlorophyll content during the tillering stage (Shen C et al., J Agric Food Chem 2025, on line). This, this fatty acid emerged as a promising, safe, and environmentally sustainable plant growth regulator for boosting wheat production in modulating auxin and gibberellin pathways.
Since 3-hydroxy fatty acids are unique structural components of the endotoxin (LPS), characteristic of Gram-negative bacteria, they can be employed as biomarkers for estimating the amount of these bacteria in atmospheric aerosols (Lee AKY et al., Atm Envir 2004, 38, 6307). Nevertheless, there are limitations in their use when analyzing animal tissues since they are also produced by mitochondrial beta-oxidation (Szponar B et al., J Microbiol Meth 2002, 50, 283). Chemically, endotoxin is lipopolysaccharides which is a major constituent of the outer membrane of Gram-negative bacteria. The lipid portion of the endotoxin, lipid A, is chemically distinct from all other lipids in biological membranes and consists of characteristic 3-hydroxy fatty acids, primarily with carbon chain lengths from 10 to 18, attached to hydroxyl and amino groups of a disaccharide backbone. It has been proposed that 3-hydroxy fatty acid quantification may be employed as biomarkers of endotoxins and Gram-negative bacterial community in atmospheric aerosols (Lee AKY et al., Atmosph Environ 2004, 38, 6307). This approach is more iformative than the determination of mass loading, total endotoxin concentration in aerosols. The relative abundance of 3-hydroxy FA in soils was shown to vary with mean annual air temperature (MAAT) and pH in soils.
3-Hydroxy fatty acids, derived from Gram-negative bacterial outer membranes, have received attention for their potential as new terrestrial pH and temperature proxies for palaeoclimate studies (Wang C., et al., Org Geochem 2016, 94, 21). It has been shown that 3-OH-FAs based proxies can also be developed and applied to the marine environment (Yang Y et al., Org Geochem 2020, 141, 103975). Further research have focused on the potential use of bacterial 3-OH-FAs as proxies for paleoclimate reconstruction in both aquatic and terrestrial environments (Yang Y et al., Org Geochem 2021, 160, 104277). A novel protocol has been proposed for extracting 3-hydroxy fatty acids from soil with microwave-assisted acid digestion (Chen T et al., Org Geochem 2025, 204, 104988).
The LPS layer is not limited to bacteria. The presence of this cell wall component has been reported in a medically important higher basidiomycete, Antrodia camphorata (Cheng J. et al., J Agric Food Chem 2005, 53, 469), this fungal LPS reversing the immuno-regulating properties exerted by bacterial LPS.
It must be noticed that 3-hydroxypalmitic acid methyl ester has been shown to be a very potent autoregulator compound controlling virulence in the phytopathogenic bacterium Ralstonia solanacearum (Flavier AB et al., Mol Bacteriol 1997, 26, 251). This fatty acid methyl ester was shown to be an intercellular signal active via a volatile phase.

3-Hydroxypalmitic acid methyl ester
It was show that 3-OH oxylipins can effect quorum sensing in Candida albicans (Nigam S et al., Curr Microbiol 2010, 62, 55), a function used by microorganisms to measure population density and to regulate pathogenicity. Thus, this yeast utilises 3-OH oxylipins, i.e. 3-OH-14:2 produced from 18:2, as a signal for expression of genes responsible for accelerating cell morphogenesis at a certain population density.
It was also found that this yeast converts arachidonic acid, released from infected host cells, to a 3-OH oxylipin (3-hydroxy eicosatetraenoic acid or 3-HETE) via incomplete mitochondrial beta-oxidation. This compound then acts as substrate for the host cyclooxygenase-2 (COX- 2), leading to the production of potent pro-inflammatory 3-OH prostaglandin E2 (Ciccoli R et al., Biochem J 2005, 390, 737).
3-Hydroxy fatty acids with two conjugated double bonds and 16 or 18 carbon atoms have been described in Fijian green macroalgae (Tydemania expeditionis) (Jiang RW et al., Phytochemistry 2008, 69, 2495). These compounds demonstrated moderate inhibitory activity against a panel of tumor cell lines (IC50 : 1.3 to 14.4 mM). Three 3-hydroxy fatty acids mainly in the phospholipids have been described in polar lipids of the alga Grateloupia turuturu (Kendel M et al., Lipids 2013, 48, 535).
An unusual unsaturated C10 fatty acid with one hydroxyl group on the carbon 6 has been isolated from the gorgonian-derived fungus Xylaria sp. C-2, collected from the South China Sea: (2E,4E,6S)-6-hydroxydeca-2,4-dienoic acid (Sun DW et al., Chemistry of Natural Compounds 2017, 53, 227).
ω-Hydroxy acids have their hydroxyl group at the methyl end of the carbon chain and can result in special glycerides with more than three acyl groups through acylation of one or more hydroxyl groups (ergot, Lesquerella and kamala oils). They participate also in the structure of suberin, a lipid polyester present in plant cell walls, and of cutin, a lipid polyester which is a component of the plant cuticle. These apoplastic structures are important plant-environment interfaces which act as barriers limiting water and nutrient loss and protecting plants from radiation and pathogens. After experimental depolymerization, monomers and oligomers containing glycerol are esterified by several hydroacids. The most frequent ω-hydroxy acids were found to be C16, C:18, C18:1, C18:2 in cutin and C18 to C24 in suberin (Graca J et al., Phytochemistry 2002, 61, 205; Graca J et al., Chem Phys Lipids 2006, 144, 96; Pollard M et al., Tr Plant Sci 2008, 13, 236). In lipid polyesters extracted from Arabidopsis and Brassica seeds, w-hydroxy acids with 16 up to 26 carbon atoms were described (Molina I et al., Phytochemistry 2006, 67, 2597).
ω-Hydroxy acids are the main building blocks of algaenan, the highly cross-linked constituent of the cell walls of green algae (Blokker P et al., Phytochemistry 1998, 49, 691). These ester-bound fatty acids are unsaturated, the monoene (n-9) having a C30, C32 or C34 carbon chain and the diene (n-18 and n-19) having a C30 and C32 carbon chain.
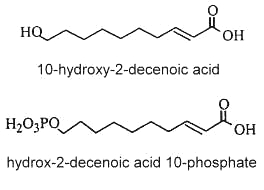
ω-hydroxy decenoic acids (known also as bee queen acid) occur in honey in small concentrations but are characteristic of the royal jelly of nurse bees (Apis mellifera). trans 10-Hydroxy-2-decenoic acid is considered as the genuine fatty compound of royal jelly (about 50 % of the total fatty acids) (Bloodworth BC, J AOAC Int 1995, 78, 1019), other hydroxylated fatty acids (10-hydroxydecanoic acid and 9-10-hydroxy-2-decenoic acid, 3,10-dihydroxydecanoic acid, 8-hydroxyoctanoic acid) have been also detected. These pheromone components which are excreted in the salivary glands of bees are said to give specific therapeutic properties to royal jelly such as skin protection, bactericide, anti-inflammatory action, immuno-regulation and anti-cancer activities. Furthermore, they could be potential candidates in the treatment of atherosclerosis (Makino J et al., J Nat Prod 2016, 79, 1137). It has been shown that worker bees secrete acids functionalized at the last (ω) position, such as 10-hydroxy-2-decenoic acid and its saturated counterpart, while the honeybee queen produces pheromones such as 9-hydroxy-2-decenoic acid, and other acids functionalized at the penultimate (ω-1) position (Plettner E et al., Science 1996, 271, 1851). Some hydroxylated fatty acids have been proposed as markers for accurate identification of the honey entomological origin (Liu X et al., Agric Food Chem 2023, 71, 7163). Important bioactivities observed for 10-hydroxydecanoic acid such as immunoregulatory, estrogenic, and anti-inflammatory activities, and in synergic combination with other molecules, have been reviewed (Gasbarri C et al., Molecules 2025, 30, 2694).
Several parent molecules have been described in the royal jelly (Noda N et al., Lipids 2005, 40, 833). It has been found that these hydroxy fatty acids strongly activate the transient receptor potential ankyrin 1 (TRPA1) and vanilloid 1 (TRPV1) (Terada Y et a., J Agric Food Chem 2011, 59, 2627). These properties could be related to the observed effects of royal jelly on several pathologies.
Besides these compounds, other fatty acids were observed : a diacid, and mono- and diesters of 10-hydroxy-2-decenoic acid in which the hydroxyl group is esterified by another fatty acid unit (estolide-like molecules). In addition, a phosphorylated derivative was also detected (2E-decenoic acid 10-phosphate). 10-Hydroxy-2-decenoic acid was shown to prevent diabetic skin dysfunction by modulating the pyroptosis pathway (Honda Y et al., Mol Nuttr Food Res 2024, 68, 2400098).
Different methods have been described for determination of 10-HDA in royal jelly, including HPLC and gas chromatography. The report of a study using HPLC and UV detection after ultrasound-assisted extraction may be consulted for literature review and technical aspects (Zhou J et al., Chromatographia 2007, 66, 185).
Due to their bifunctional nature, w-hydroxy fatty acids are used in various industrial products ranging from pharmaceuticals, cosmetics, coatings, surfactants and general polymer building blocks (Metzger JO et al., Appl Microbiol Biotechnol 2006, 71, 13). The biological production of w-hydroxy fatty acids represents an emerging biotechnology (Bitto NJ et al., Lipid Technol 2009, 21, 216).
ω–Hydroxy octadecanoic acid is showing emerging therapeutic potential in cancer biology (Kokotou MG et al., J Med Chem 2020, 63, 12666).
ω-Hydroxy octadecenoic acid : kamlolenic acid with the following structure, 18-hydroxy, 9c, 11t, 13t-18:3, is found in kamala oil, a product extracted from the seeds of Kamala tree (Mallotus phillipinensis, Euphorbiaceae) (Calderwood RC et al., J Sci Food Agric 1954, 5, 382). The kamala oil can be used as a substitute for tung oil, obtained from Aleurites spp., in the production of rapid-drying paints and varnishes. The seed oil is also used as a fixative in cosmetic preparations. The oil is also used as a fixative in cosmetic preparations and for coloring foodstuffs and beverages.
A large array of hydroxylated fatty acids deriving from linoleic acid, α-linolenic acid, roughanic acid (16:3 n-3), and other C20 fatty acids (arachidonic acid, EPA), mainly under the action of lipoxygenase, are named Oxylipins (Mosblech A et al., Plant Physiol Biochem 2009, 47, 511). The previously formed hydroperoxides are subsequently transformed by the action of different enzymes into these hydroxylated fatty acids but also into oxo fatty acids, divinyl ethers, volatile aldehydes, and jasmonates. They are widespread in nature occurring in plants, mosses, algae, bacteria, fungi and sometimes in animals. In general, oxylipins are bioactive metabolites involved in regulating developmental processes and in environmental and pathological responses.
Very-long-chain fatty acids (C28–C34) containing a hydroxy group at the n-18 position have been identified in the microalgae from the genus Nannochloropsis (Gelin F et al., Phytochemistry, 1997, 45, 641). That constant position likely indicates that the series results from chain-elongation of a particular hydroxy fatty acid. Several hydroxylated fatty acids with 16 and 18 carbon atoms have been described in microalgae, the most abundant being hydroxylated on the C11, C8, and C13 (de los Reyes C et al., Phytochemistry 2014, 102, 152). These compounds are frequently inhibitors of the TNF-a production. Thus, the most active oxylipin was a C-16 hydroxy acid, which at 25 mM caused a 60% decrease of the TNF-a level in LPS-stimulated macrophages.
An oxylipin, 15-hydroxylinoleate (avenoleic acid) has been isolated from seeds of oat (Avena sativa) and was named avenoleic acid (Hamberg M et al., Phytochemistry 1996, 42, 729). That fatty acid seems specific for oat, as it was not detectable in seeds of barley, rye, or wheat. Further studies have shown that avenoleic acid was found to be mainly localized in the glycolipid fraction of oat seed lipids (Hamberg M et al., Lipids 1998, 33, 355).
Among the 12-hydroxy acids, the most abundant is ricinoleic acid ((9Z,12R)-12-Hydroxyoctadec-9-enoic acid) which characterizes castor oil (from Ricinus communis). It was discovered in 1848 (Saalmüller L, Ann 1848, 64, 108). Goldsobel AG (Ber 1894, 27, 3121) showed that ricinoleic acid has the actual molecular structure. This acid is the only one hydroxylated fatty acid used in oleochemical industry. The seed oils of Jatropha gossypifolia and Hevea brasiliensis(Euphorbiaceae living in South America and India) were found to contain high content of ricinoleic acid (about 18%).
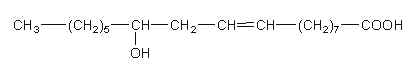
Ricinoleic acid is abundant in castor oil (90%) but many common vegetable oils and oil seeds contain lower amounts of that particular fatty acid. Thus, its content amounts to 0.27% in cottonseed oil, 0.03% in soybean oil, and 0.02% (Yamamoto K et al., Lipids 2008, 43, 457).
While known chiefly as a purgative, few decades ago, this fatty acid affords now a wide range of reactions enabling the formation of several derivatives. These chemicals are petrochemical products for use in several industrial applications. Castor oil and its derivatives are used in food (additive), textile (surfactants, pigment wetting agents), paper (defoamer, water proofing additive), plastics (Nylon-11, polyamide resin known as Rilsan 11 used for coating metals, plasticizers, coupling agents, tubes, films), perfumes and cosmetics (emulsifiers, deodorant), electronics (capacitor fluids, polyurethane and polyamide resins), pharmaceuticals, paints, inks, adhesives and lubricants. Castor oil is also used to make emulsifier after transesterification of fatty acids from the glycerol to the hydroxyl group in ricinoleic acid and ethoxylation to give castor oil polyethylene glycol (Diehl B, Lipid technol 2011, 23, 278). The laxative action of castor oil and ricinoleate is brought about by direct physiological effects that appear to involve several signaling pathways including NO, platelet-activating factor, and eicosanoids (Gaginella TS et al., Phytotherapy Res 1998,12 (Suppl. 1), S128–S130).
The production of conjugated linoleic acid by dehydration and isomerization of ricinoleic acid has been described (Villeneuve P et al., JAOCS 2005, 82, 261).
Castor oil can be reacted with sulfuric acid to make Turkey-Red Oil, the first synthetic detergent or surfactant after ordinary soap, a predecessor to sodium lauryl sulfate. These properties are the result of the sulfonation of ricinoleic acid. Turkey-Red Oil is also used in the dyeing of cotton texture.
The limited amount of castor oil as natural source of ricinoleic acid has led chemists to develop suitable processes for the preparation of hydroxy fatty acids from commercial plant oils (Dahlke B et al., JAOCS 1995, 72, 349). The microbial transformation of ricinoleic acid with the yeast Yarrowia lipolytica yields g-decalactone, an aroma compound with fruity and oily notes found naturally in fruits and fermented foods ( Schrader J. et al., Biotechnol Lett 2004, 26, 463).
A fatty acid isomeric with ricinoleic acid, 9S-hydroxy-12Z-Octadecenoic acid (strophantus acid), has been shown to be of general occurrence (between 9 and 15%) in the seed oils of the genus Strophantus (Apocynaceae) (Gunstone FD et al., J Sci Food Agric 1959, 10, 522) but was also found in Holarrhena, Nerium and Wrightia of the same family. It was reported for the first time in the seed oil of the desert rose Adenium obesum in which it is present at a level of around 26 % (Smith MA et al., JAOCS 2016, 93, 105).
This fatty acid is a potential renewable feedstock for the oleochemical industry, mainly as a precursor for the synthesis of antimicrobial compounds.
Ricinelaidic acid or (9E,12R)-12-Hydroxyoctadec-9-enoic acid is an unsaturated omega-9 trans fatty acid. an isomer of ricinoleic acid. Investigations have revealed that this fatty acid is a LTB4 receptor antagonist, which may account in part for their reported anti-inflammatory activities (Yagaloff KA et al., Prost, Leukotr Essential Fatty Acids 1995, 52, 293).
As castor seed production presents some problems (toxicity of the seed, allergic reactions), Lesquerella species were proposed as a valuable source in the USA (up to 70% in the oil) of ricinoleic acid but also of lesquerolic acid, the C20 homologue of ricinoleic acid (14-hydroxy-11-eicosenoic acid). One of the most studied species is Physaria fendleri, formerly Lesquerella fendleri, Brassicaceae. That plant is a new industrial oilseed crop in the southwestern region of the U.S. can also be used for industry similar to those of ricinoleate and it is amenable to Agrobacterium-mediated transformation to increase the lesquerolic acid production (Wang W et al., Plant Cell Tissue Organ Cult 2008, 92, 165). The identification of triacylglycerol and diacylglycerol species in the seed oil of Physaria fendleri using mass spectrometry has been reported (Lin JT et al., J Am Oil Chem Soc 2013, 90, 1819). In these species, two other hydroxylated fatty acids are found : densipolic acid (12-hydroxy-9,15-octadecadienoic acid) and auricolic acid (14-hydroxy-11,17-eicosadienoic acid) (Smith CR et al., J Org Chem 1962, 27, 3112). Densipolic acid is present also in Paysonia species (Brassicaceae) and in triacylglycerols of Lesquerella lyrata (Brassicaceae) and forms estolide complexes (tetra-acylglycerols) when esterified by another normal fatty acid (triacylglycerol-estolides) (Zhang H et al., Ind Crops Prod 2012, 37, 186). Thirteen tetra-acylglycerol species have been detected in Physaria fendleri (Lin JT et al., J Am Oil Chem Soc 2013, 90, 1831).
Analyses have indicated the presence of densipolic acid and its trans-isomer in various edible oils (Plitzko K et al., Food Chem 25 August 2025, 146091). The highest concentrations were found in n3-PUFA rich oils (flaxseed and shiso oil) and lower concentrations in rapeseed and soybean oils.
It has been suggested that these compounds could be formed by the enzymatic desaturation of ricinoleic acid via fatty acid desaturase 3 (Engeseth N et al., Planta 1996, 198, 238).
11-Hydroxy hexadecanoic acid, or jalapinolic acid, occurs in jalapin (resin from the rhizoma of Ipomea operculata) and in scammoniae resina (resin from the roots of Convolvulus scammonia). The main components containing jaliponoic acid in these resins are operculinic acids (Ono M et al., Chem Pharm Bull 1990, 38, 2650).
10-hydroxy-cis-12-octadecenoic acid, a gut microbial metabolite of linoleic acid, was shown to ameliorate intestinal epithelial barrier impairment partially via GPR40-MEK-ERK pathway (Miyamoto et al., J Biol Chem 2015, 290, 2902). In contrast, 10-hydroxyoctadacanoic acid, which is a gut microbial metabolite of oleic acid did not show these tight junction-restoring activities and down-regulated GPR40 expression.
Hydroxystearic and hydroxypalmitic acids, consisting of isomers with the hydroxyl group at different positions, were identified in milk. Among them those carrying the hydroxyl at the 7- and 9-positions presented growth inhibitory activities against various human cancer cell lines (Kokotou MG et al., J Med Chem 2020, 63, 12666).
9-Hydroxyacid : another unusual hydroxylated fatty acid which may have many potential oleochemical applications has been discovered in munch seed oil (Dimorphotheca pluvialis) : β-dimorphecolic acid (9-hydroxy,10t,12t-18:2) (Smith CR et al., J Am Chem Soc 1960, 82, 1417). This compound which is also a conjugated diene seems to be a versatile raw material for applications in chemical, pharmaceutical, flavor and fragrance industries since it can be readily dehydrated to a mixture of conjugated triene acids. The 10t12c form of the 9-hydroxy-18:2 (isomeric with dimorphecolic acid) is present in the seed oil of Xeranthemum annuum, Dimorphotheca, Tragopogon, Tagetes, Bidens and Cosmos. This compound was shown to be a partial agonist at PGEl and PGD2 receptors on human platelets (Henry DY et al., Eur J Biochem 1987, 170, 389).
Dimorphecolic acid has been isolated in relatively pure state by supercritical carbon dioxide extraction of munch seed oil (Cuperus FP et al., JAOCS 1996, 73, 1675).
Another isomeric form of dimorphecolic acid, coriolic acid (13-hydroxy-9c,11t-octadecadienoic acid), was reported to be present at high level (70%) in the seed oil of a Coriaraceae Coriaria myrtifolia (Hanseen KS et al., Acta Chem Scand 1967, 21, 301) but also in a Polygonaceae Monnina emerginata (30%) (Phillips BE et al., Biochim Biophys Acta 1970, 210, 353).
Unsaturated oils can contain small amounts of hydroxy derivatives on storage, probably through enzymatic and/or non-enzymatic oxidation.
A wide range of hydroxylated fatty acids is found in sediments but unfortunately these compounds have received little attention from organic geochemists. Long-chain up to C24 and a- and b-monohydroxy acids were observed in a 5000 year-old lacustrine sediment from the English Lake district (Eglinton G et al., Tetrahedron 1968, 24, 5929), and were attributed to microbial oxidation of monocarboxylic fatty acids.
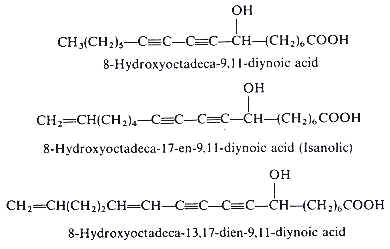
Analytical works on the seed oil of Ongokea gore (Olacaceae) have demonstrated the existence of a variety of hydroxylated diynoic acids (review in Badami RC et al., Prog Lipid Res 1981, 19, 119). Some of them have no other double bond as 8-hydroxyoctadeca-9,11-diynoic acid, others have in addition one double bond as isanolic acid or two double bonds as 8-hydroxyoctadeca-13,17-dien-9,11-diynoic acid.
Five novel di-hydroxylated fatty acids, cladosporacids A–E were isolated from halotolerant fungal strains of Cladosporium cladosporioides isolated from mangrove (Rhizophora stylosa) roots in Shankou, Guangxi Province, China (Peng X et al., Reson. Chem., 2018, 56, 18).
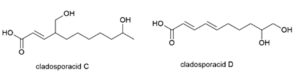
Two cladosporacids
Pseudomonas aeruginosa has been intensively studied to produce a novel 7,10-dihydroxy-8(E)-octadecenoic acid from oleic acid and natural vegetable oils containing oleic acid. It was shown to have antibacterial activities against food-borne pathogenic bacteria but also anti fungal activities (Kim HR et al., J Oleo Sci 2024, 73, 215).
A long-chain dihydroxy fatty acid has been found in an Euphorbiaceae Baliospermum axillare seed oil and characterized as 11,13-dihydroxy-tetracos-9t-enoic acid (Husain S et al., Phytochemistry 1980, 19, 75). It was named axillarenic acid.
Two dihydroxy fatty acids have been isolated from floral oils produced by various flowers (Mariza R et al., J Chem Ecol 2007, 33, 1421). These fatty acids (3,7-dihydroxy-eicosanoic and docosanoic acids) were named tetrapedic acids. One of them (3,7-dihydroxy-docosanoic acid) may be di-acetylated, and was named byrsonic acid. These fatty acids were also found acylating a mono-acylated glycerol molecule. These oily compounds are produced by special flower devices (elaiophores) and attract pollinating insects.
Two dihydroxy fatty acids, 15,16-dihydroxy-30:0 and 16,17-dihydroxy-33:0, have also been identified from the acid hydrolysis of the cell residue of Nannochloropsis.
The seed oil of Cardamine impatiens (Cruciferae, Brassicaceae) contains a series of long-chain vicinal dihydroxy fatty acids which make up of about 25% of the oil (Mikolajczak KL et al., JAOCS 1965, 42, 939). This peculiar structure remains unique in phytochemistry. One example with a C18 chain is shown below. Other members of the series have a C20, C22 or a C24 chain but with the hydroxyl groups remaining at the same position with respect to the terminal methyl group. 9,10-Dihydroxyoctadecanoic acid was also found at 11% in the seed oil of a Rutaceae Feronia elephantum (Badami RC et al., J Oil Tech Assoc India 1972, 4, 59).
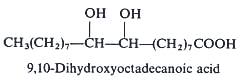
An isomeric form of the previous one but not with vicinal hydroxy groups has been described in the seed oil of a Rutaceae Peganium harmala, 9,14-dihydroxyoctadecanoic acid (Ahmad I et al., Phytochemistry 1977, 16, 1761).
Among hydroxylated derivatives of oleic acid, it has been shown that 8,11-dihydroxyoctadec-9Z-enoic acid exhibited significantly greater Ca2+ response in the GPR40/GPR120-expressing cells as compared to the endogenous agonists (e.g., linoleic acid and docosahexaenoic acid). It was shown that not only the degree of unsaturation but also the number and position of hydroxyl groups played a key role in their agonist activities (Park Y et al., Food Chemistry 2024, 142010).This study will contribute to understanding the relationships between fatty acid structures and agonist activities.
Cutin is known to be a polyester polymer which is insoluble and can be hydrolyzed mainly into a mixture of long-chain C16 and C18 ω-hydroxyacids having frequently hydroxyl or epoxy groups in secondary positions. Phellonic acic, 22-hydroxydocosanoic acid, was also detected in cork suberin. Several studies led to tentative models of cutin based on the inter-esterification of ω-hydroxyacids, both head-to-tail in a linear form , and cross-linked via the secondary hydroxyls. In some plant species cutin, 16-carbon dihydroxy and 18-carbon trihydroxyacids were detected (Graca J et al., Phytochemistry 2002, 61, 205).
Suberin is a similar type of polyester polymer which contains among other fatty acids α,ω-diacids as long-chain monomers esterifying glycerol (Graca J et al., Chem Phys Lipids 2006, 144, 96). Moreover, these diacids (C16, C18, C18:1, and C22) are further esterified by another glycerol molecule or by a hydroxylated fatty acid.
A trihydroxylated oxo-fatty acid, phaseolic acid (2-oxo-5,8,12-trihydroxydodecanoic acid) was shown to stimulate elongation in pea stem segments (Farmer EE, Plant Mol Biol 1994, 26, 1426). This compounds is reminiscent of animal lipoxins.
The 9,10,18-trihydroxyoctadecanoic acid (phloionolic acid) was isolated from the seed oil of Chamaepeuce afra (Mikolajczak KL et al., Lipids 1967, 2, 261) but was also recognized as an important constituent of cork suberin (Holloway PJ, Chem Phys Lipids 1972, 9, 158).
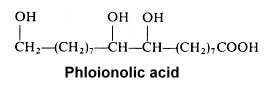
A monounsaturated derivative of phloionolic acid was also detected in some seed oil.
Five trihydroxylated fatty acids with 2 or 3 double bonds (Trichoderols) have been isolated from the culture of Trichoderma sp. Z43 obtained from the surface of the marine brown alga Dictyopteris divaricata (Shi ZZ et al., Mar Drugs 2023, 21, 453). All compounds displayed moderate antimicroalgal activity with IC50 ≥ 15 μg/mL and low toxicity to the brine shrimp Artemia salina.
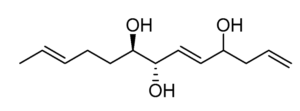 Trichoderol
Trichoderol
A new tetrasubstituted octanoic acid, named hyfraxinic acid, was isolated from the organic extract of Hymenoscyphus fraxineus responsible for ash (Fraxinus excelsior L.) dieback in Europe (Mais M et al., J Agric Food Chem 2019, 67, 49, 13617). Hyfraxinic acid was characterized, using spectroscopic methods, as 2,4-dihydroxy-7-methyl-6-methyleneoctanoic acid. It exhibited remarkable phytotoxicity on several plants tested, inducing necrotic lesions at low concentrations.
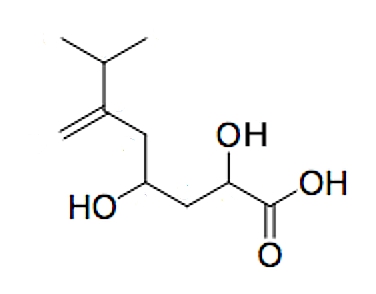
Hyfraxinic acid
Higher plant cutin and suberin can also be a significant source of esterified C16-C22 α-, β-, and ω-monohydroxy and C16 and C18 polyhydroxylated fatty acids in sediments (Cardoso JN et al.,Geochim Cosmochim Acta 1983, 47, 723).
Estolides are dimers formed by a fatty acid ester of hydroxy fatty acid. They are also named branched fatty acid esters of hydroxy fatty acids. They are also found in some special triglycerides where they acylate the sn-3 position and formed estolide tetraester triglyceride. Two structurally and functionally distinct estolide superfamilies are recognized based on the position of the estolide bond: omega-estolides and in-chain branched estolides. The existing variety of possible hydroxy fatty acids and fatty acids combined with the position of the estolide bond generates a vast quantity of unique structures identified in estolides families.
Estolides were discovered for the first time in mammals as important signaling molecules with potential anti-inflammatory and anti-diabetic effects.Thus, they are potential therapeutic agents for type 2 diabetes and inflammatory diseases like obesity (Yore MM et al., cell 2014, 159,318 ; Li L et al., Obes Rev. 2024, 25(6), e13735). It appears that higher maternal BMI was associated with higher concentrations of 2 saturated estolides and a lower concentration of 1 polyunsaturated estolide in breast milk. Specific estolides isomers were associated with growth and body composition development in exclusively breastfed infants during early infancy. It has been demonstrated that 5-palmitic acid ester of hydroxystearic acid contained in breast milk played a role in macrophage foam cell formation (Zhang Y et al., J Agric Food Chem October 8, 2025). In an oxidized low-density lipoprotein-induced foam cell model, that estolide reduced intracellular lipid accumulation, decreased the cholesterol ester/total cholesterol ratio and upregulated cholesterol efflux/hydrolysis genes.
A review of their putative healthy properties has been published (Riecan M et al., Pharmacol Ther. 2022, 231, 107972). Results have suggested that docosahexanoic acid-derived estolide could be a novel and potent activators of NRF2 with plausible antioxidant function (Gowda S et al., Int J Mol Sci 2021, 22, 7598).
Currently, 583 estolides have been identified in the human white adipose tissue (Brejchova K et al., Prog Lipid Res 2020, 79, 101053).
Estolides are found in seed oil from Euphorbiaceae. A hydroxy allenic acid (8-hydroxy-5,6-octadienoic acid) was described in an estolide from Sebastiana commersoniana (Spitzer V. et al. Lipids 1997, 32, 549). The ω-hydroxyl group was shown to be acylated by a conjugated diene with 10 carbon atoms.
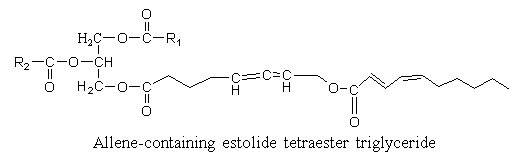
The allenic acid was shown to have antifungal properties (Ohigashi H et al Agr Biol Chem 1972, 36, 1399), the triglyceride being not recommended for animal diet.
The first biologically active estolides were discovered in the glandular hairs of larvae of the European cabbage butterfly (Pieris rapae) and named mayolenes in honor of May Berenbaum, an American entomologist (Smedley SR et al., PNAS 2002, 99, 6822).
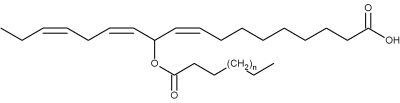
Mayolenes (n = 9 to 15)
These estolides are formed by a 11-hydroxylinolenic acid esterified by a saturated fatty acid (C14-C20). Bioassays demonstrated that they are potent deterrent, playing a defensive role against predators.
Various natural-origin estolides have been detected in different teas (Zhu QF et al., J Agric Food Chem 2022, 70, 5369). The estolide contents had a positive relationship with the tea fermentation degree. The abundance of some of these molecules might be at the origin of the anti-diabetic function of teas.
Classical wax esters were found and based on C18:n (n = 1–4) unsaturated fatty acids ranking in the following order of abundance: C18:1>C18:2>C18:3>C18:4, the major fatty alcohols being of saturated nature and varied from C18:0 to C28:0.
Lipidomic analysis of mouse adipose tissue revealed the existence of estolides that were elevated 16- to 18-fold in these mice. Several isomers differ by the branched ester position on the hydroxy fatty acid (e.g., palmitic acid-9-hydroxystearic acid). These estolides are synthesized in vivo and regulated by fasting and high-fat feeding. Their level correlates highly with insulin sensitivity and are reduced in adipose tissue and serum of insulin-resistant humans, These compounds could have the potential to treat type 2 diabetes (Yore MM et al., Cell 2014, 159, 318).
Estolides are found in mammalian serum and tissues as non-esterified molecules at low concentrations. The concentrations of estolide-containing triacylglycerols in adipose tissue were reported to be 100-fold greater than that of non- esterified estolides, suggesting that these triglycerides may serve as a major reservoir of these lipids (Tan D et al., J Am Chem Soc 2019, 141, 22, 8798).
The secretions of the human Meibomius glands (meibum) which are mixed with tears, contain also estolides based on a ω-hydroxylated fatty acid (mainly from 30 to 34 carbons) acylated on the terminal hydroxyl by oleic acid (18:1n-9) (Butovich IA et al., J Lipid Res 2009, 50, 2471). Due to their amphiphilic anionogenic nature, these compounds may be responsible for stabilization of the tear film lipid layer.
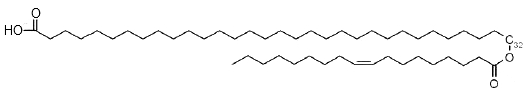
ω-Estolide from meibum
An overview of the diversity of estolides, nomenclature, and their biological effects is provided (Brejchova K et al., Prog Lipid Res 2020, 79, 101053).
Industrially, estolides are now synthesized from vegetal oils and are used as ingredients in various industrial fields. Thus, these new functional fluids have a rapidly growing importance in cosmetics, coatings, and biodegradable lubricants.
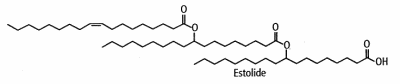
They are largely synthesized from oleic acid warmed with perchloric or sulfuric acid (Cermak SC et al. JAOCS 2001, 78, 557). The average number of fatty acid units added to the first base fatty acid (named “estolide number”) varied as a function of reaction temperature. The secondary ester linkages are more resistant to hydrolysis than those of triglycerides, and the unique structure of the estolide results in materials having far superior physical properties than mineral oils and vegetable and petroleum-based oils. They are said to improve intra-fiber moisture retention, to restore elasticity, and prevent mechanical damage. In skin care systems, they provide significant moisturization benefits.
Estolides made from vegetal oils have a good oxidative stability and low-temperature properties. Oxidative stability may be improved in removing the unsaturation of oleic acid and the low-temperature performance may be improved in using oleic acid and various short or middle-chain saturated fatty acids (lauric or myristic acid, coconut oil). Other chemical developments are in progress to obtain molecules with required functional fluid conditions (Cermak S et al., Inform 2004, 15, 515). The synthesis of estolides from fatty acids using four different types of catalysts, namely, mineral acids, solid acids, lipases, and ionic liquids have been reviewed (Chen Y et al., JAOCS 2020, 97, 231).
The analysis of these compounds may be effected by a combination of gel permeation chromatography, TLC, and gas chromatography (Fehling E, JAOCS 1995, 72, 355).
A kind of estolide has been described in the stem bark of an Euphorbiaceae (Alchorena laxiflora), a plant used in Africa to treat some diseases (Sandjo LP et al., JAOCS 2011, 88, 1153). That compound is formed of a C14 fatty acid with a double bond in the n-3 position esterified with a hydroxylated propionic acid.
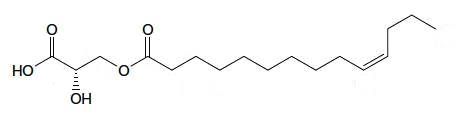
Estolide from Alchorena
Macrolactins are macrolides containing three separate diene structure elements in a 24-membered lactone ring which were first reported to be produced by several marine bacteria (Gustafson K et al., J Am Chem Soc 1989, 111, 7519). Several macrolactin structures have been described in marine bacteria, the simplest one (macrolactin A) is shown below.
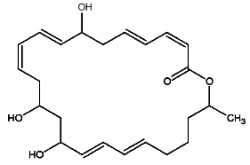
Macrolactin A
These antibiotics may be considered equivalent to a tetrahydroxylated tetracosaenoic acid with an ester bond between the carboxyl group and one of the hydroxyl groups (lactone structure). They are considered to be potent antiviral (against herpes and HIV) and cytotoxic (against melanoma) agents that also have antibacterial activity. It was suggested that the hydroxyl group at C-15 may play an important role in the antibacterial activity of these compounds. There is yet no information about the mechanism of action of this group of compounds.
Parcoblattalactone is a macrocyclic lactone deriving from a 12-carbon fatty acid hydroxylated in position w. This compound is a sex pheromone of the insect Parcoblatta lata, which represents the most abundant arthropod biomass in the pine forests of the southeastern United States (Eliyahu D et al., PNAS, 2012, 109, 490-6). This pheromone will be used for monitoring populations of insects that comprise an important food source for endangered bird species.
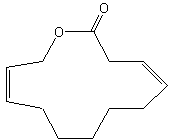
Parcoblattalactone
![]()
LIPOXYGENASE PRODUCTS
Lipoxygenase activities give rise to important hydroxylated derivatives mainly from polyunsaturated fatty acids.
Thus, lipoxygenation of 20:4(n-6) results in the formation of a variety of mono- and dihydroxy derivatives. The monohydroxy derivatives consist of the positional isomers 5-hydroxy-20:4, 12-hydroxy-20:4 and 15-hydroxy-20:4. The dihydroxy derivatives include products arising from 5- or 15-lipoxygenation. Double lipoxygenation of 20:4(n-6) at c5 or c12 position give rise to 5,12-diHETE.
In addition to 20:4(n-6), linoleic acid (18:2n-6) is also a substrate for lipoxygenases. In leucocytes (neutrophiles), 13-hydroxy-18:2 (coriolic acid) is produced through enzymatic activity. It appears to be also produced by vascular endothelial cells where it prevents platelet attachment and has vasoconstrictor activity.
It has been shown that linolenic acid (18:3n-3) may be converted by 15-lipoxygenase into mainly 13(S)-OH-18:3 after reduction of the hydroperoxide product. But 18:3n-3 leads also to four di-hydroxylated fatty acid isomers. These compounds are: 9(R),16(S)-dihydroxy-10 E,12 E,14 E-octadecatrienoic acid, 9(S),16(S)-dihydroxy-10 E,12 E,14 E-octadecatrienoic acid, 9(S),16(S)-dihydroxy-10 E,12 Z,14 E-octadecatrienoic acid, and 9(R),16(S)-dihydroxy-10 E,12 Z,14 E-octadecatrienoic acid (Liu M et al., J Lipid Res 2013, 54, 2083).
The double oxygenation of ALA by 15-lipoxygenase produced oxylipins were called linotrins. They possess two hydroxyl residues and an unsaturation zone characterized by three in series conjugated double bonds having the E,Z,E cis-trans configuration. Two main isomers have been identified: 9S,16S-10E,12Z,14E-octadecatrienoic acid (linotrin-1) and 9R,16S-10E,12Z,14E-octadecatrienoic acid (linotrin-2). Interestingly, 9,16-dihydroxy-10 E,12 Z,14 E-octadecatrienoic acid isomer exhibits anti-aggregatory properties as other dioxygenated derivatives of PUFA containing an E,Z,E-conjugated triene (PUFA oxygenated trienes or poxytrins) (Liu M et al., J Lipid Res 2013, 54, 2083). These omega-3 oxylipins featuring an E,Z,E conjugated triene motif were shown to be present in the plant kingdom and alleviate inflammation in LPS-challenged microglial cells (Balas L et al., Eur J Med Chem 2022, 231, 114157).
Allium species (onion and garlic) which are known as folk medicine for the treatment of atherosclerosis and some ulcers, were shown to be rich in two trihydroxylated derivatives of 18:2(n-6) : 9,10,13- and 9,12,13-trihydroxy octadecenoic acids. Furthermore, it was shown that these products have PGE-like activity in in vitro bio-assay tests (Claeys M et al., Prog Lipid Res 1986, 25, 53). Similar products were isolated from roots of Bryone ala, used also for similar medicinal purposes as onion (Panossian AG et al., J Med Plant Res 1983, 47, 17).
Lipoxygenase derivatives of docosahexaenoic acid (DHA), named docosanoids, are known to be formed (mainly 11-OH-DHA) in retinal cells (Bazan N et al., Biochem Biophys Res Comm 1984, 125, 741), but their exact structure and bioactivity were revealed only after 2002. A review of their formation and function in blood and vascular cells may be consulted (Lagarde M, Eur J Lipid Sci Technol 2010, 112, 941). A critical overview on the dihydroxy-docosatrienes may be also consulted (Balas L et al., Biochimie 2014, 99:1-7) and their roles in metabolic diseases has been also reviewed (Spite M et al., Cell Metab 2014, 19, 21).
Thus, lipidomic analysis of exudates, vascular, leukocytes and neural cells treated with aspirin have revealed hat DHA was converted into 17R-hydroxy series of dihydroxy- and trihydroxy-docosanoids termed “resolvins” (D-series). They are formed during the resolution phase of acute inflammatory response and are able to counter proinflammation signals (Serhan CN et al., J Exp Med 2002, 196, 1025; Serhan CN et al., Prost Lipid Med 2004, 73, 155). Several oxygenase enzymes may be responsible for metabolizing polyunsaturated fatty acids to resolvins: 15-lipoxygenase-1, possibly 15-lipoxygenase-2, 5-lipoxygenase, cyclooxygenase-2 (COX-2), and certain Cytochrome P450 monooxygenases.
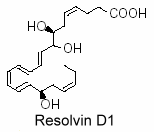
A novel resolvin, 7S,15R-dihydroxy-16S,17S-epoxy-docosapentaenoic acid was shown to be synthesized by cyanobacterial lipoxygenase from DHA. It has been demonstrated that this derivative was able to inhibit colorectal cancer stemness through repolarization of tumor-associated macrophage functions (Wang LF et al., Antioxidants 2021, 10, 1459).
Similarly, EPA is converted into a dihydroxy derivative (resolvin E2) and a trihydroxy derivative (resolvin E1) with five double bonds (E-series) (Oh SF et al., Biochim Biophys Acta 2011, 1811, 737-747).
The metabolism and pharmacological functions of these resolvins have been reviewed (Serhan CN et al., Brit J Pharmacol 2008, 153, S200).
On another hand, brain ischemia was shown to induce a release of DHA from membrane phospholipids which then generates via enzymatic oxygenations novel derivatives named “docosatrienes“. These dihydroxy-containing DHA derivatives were termed “neuroprotectins“.
The main member of the series was 10,17S-docosatriene (neuroprotectin D1) which was proved to be a potent regulator of inflammation (Marcheselli VL et al., J Biol Chem 2003, 278, 43807).
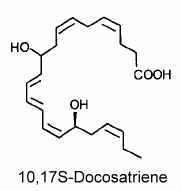
Neuroprotectin D1
Several isomers of protectin D1 were synthesized using soybean lipoxygenase and tested for their ability to inhibit human blood platelet aggregation. It was discovered that the oxygenated products having the E,Z,E-conjugated triene motif and collectively named poxytrins (PUFA oxygenated trienes), might have potent antithrombotic potential (Chen P et al., FASEB J 2011, 25, 382).
The biosynthetic pathway of that DHA derivative in retinal pigment cells and its protective effects from apoptosis induced by an oxidative stress were reported (Mukherjee PK et al., PNAS 2004, 101, 8491). These compounds may be the basis of new therapeutic approaches to enhance photoreceptor survival in retinal degenerations. A review of the rescue and repair processes during photoreceptor cell renewal mediated by neuroprotectin D1 may be consulted (Bazan NG et al., J Lipid Res 2010, 51, 2018).
Some isomers exhibiting the 11t,13c,15t geometry, instead of 11t,13t,15c as in protectin D1 (poxytrins family), are able to inhibit strongly blood platelet aggregation (Chen P et al., FASEB J 2011,25, 382).
Besides 10,17S-docosatriene, the analogue compound 7,17S-docosatriene was shown to be produced during aerobic oxidation of DHA by soybean lipoxygenase (Butovich IA et al., J Lipid Res 2006, 47, 2462). Enzymatic investigations suggest that these compounds might have anti-inflammatory and anticancer activities, which could be exerted, at least in part, through direct inhibition of 5- and 15 lipoxygenase. An overview of their role in brain physiology and a discussion on the potential of using DHA signaling in the development of treatments in patients suffering from stroke have been released by Niemoller TD et al. (Prost Lipid Mediat 2010, 91, 85).
One neuroprotectin and several resolvins have been shown to be biosynthesized by isolated trout brain cells providing the first evidence for the conservation of these structures from fish to humans as chemical signals in diverse biological systems (Hong S et al. Prost Lipid Mediat 2005, 78, 10).
Maresin 1 (7,14-dihydroxydocosa-4 Z ,8,10,12,16 Z ,19 Z -hexaenoic acid) is a new lipoxygenase product from DHA produced in macrophages. It appears as potent as neuroprotectin D1 in its anti-inflammatory activity (Serhan C N et al., J Exp Med 2009, 206, 215).

Maresin 1
Vascular endothelial cells treated with aspirin was shown to convert eicosapentaenoic acid (EPA) into an intermediate product which gives a bioactive compound 5,12,18R-trihydroxy-EPE (resolvin E1).
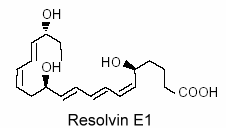
This resolvin was shown to be a potent regulator of PMN cells and inflammation (Serhan CN et al., Prost Lipid Med 2004, 73, 155). New resolvins derived from docosapentaenoic acid of the n-6 family (22:5n-6) have been characterized (Dangi B et al., J Biol Chem 2009, 284, 14744). These products resulting from 15-lipoxygenase activity were determined to be potent anti-inflammatory agents.
A comprehensive review of the metabolism and properties of resolvins, docosatrienes and neuroprotectins may be consulted (Serhan CN et al., Lipids 2004, 39, 1125). An overview of their synthesis and their biological significance have been reviewed (Balas L et al., Prog Lipid Res 2016, 61, 1-18). A review of the importance of polyunsaturated fatty acids and their oxygenated or hydoxylated metabolites in the blood vessel compartment may also be consulted (Lagarde M et al., Prog Lipid Res 2015, 60, 41).
Hypoxilins, hydroxy-epoxy derivatives of arachidonic acid are described with lipoxygenase products.
DISPERSIVE LIQUID-LIQUID MICROEXTRACTION
Lire la suiteDevenez membre et participez au développement de la Lipidomique au XXIème siècle.
S'inscrire