
ANALYSIS OF LONG-CHAIN BASES
To obtain the long-chain bases from sphingolipids, it is first necessary to hydrolyze phosphate, glycosidic or amide bonds by an efficient procedure before the products are isolated and purified. These bonds can be cleaved simultaneously by reaction with acid run at high temperature.
The solution of free long-chain base obtained by acid hydrolysis may be used to:
1- estimate the total amount of long-chain bases
2- separate the various classes of bases
3- separate and quantify the long-chain bases by :
As all glycosphingolipids and sphingomyelin contain one mole of long-chain base, the total amount of these lipids can be appreciated by the amount of fatty bases present in the hydrolyzed extract.
Several procedures may be used but we propose a very simple one giving easily reliable and accurate data. The quantitative procedure is base on the colorimetric or fluorometric estimation of derivatized long-chain bases.
![]()
1 – Quantification of the total long-chain base pool
A – Spectrophotometric method
Material and Reagents:
Spectrophotometer (340 nm)
Sodium bicarbonate, trinitrobenzenesulphonic acid (TNBS)
1M HCl in methanol, hexane, ethanol
Procedure:
– The solution containing the long-chain bases, after their hydrolysis from ceramides, is evaporated and 1 ml 4% (w/v) sodium bicarbonate in water is added with 1 ml 1% (w/v) TNBS in water. Keep in the dark for 1 h at 40°C.
– Add 1 ml of methanolic HCl solution and extract two times long-chain bases with 2 ml hexane. After evaporation of hexane, samples are dissolved in 2 ml ethanol and the absorbance of the solution is obtained at 340 nm relative to an appropriate blank. The amount in an unknown solution is read from a calibration curve prepared with pure commercial standards.
Another practical approach to the technical problem of the hydrolysis of ceramides has been described using a one-spot heating in a household microwave oven with 0.1 M NaOH in methanol for 2 min followed by 1M HCl in methanol for 45 s (Itonori S et al., J Lipid Res 2004, 45, 574).
B – Fluorometric method
Material and Reagents:
Fluorometer, glass cuvettes, 0.2 M sodium borate buffer pH8, 2 M NaOH in water, diethyl ether, fluorescamine
Procedure:
After the hydrolysis of ceramide-containing lipids, add to the solution (0.5 ml) containing the long-chain bases 0.75 ml of borate buffer and 0.25 ml of 2 M NaOH, and mix. Add 1.5 ml of diethyl ether, then add 0.5 ml of fluorescamine (0.15 mg/ml in diethyl ether). Cap the tubes and vortex vigorously for 1 min. Centrifuge 5 min at low speed and transfer the upper phase to a glass cuvette. Measure the fluorescence (Exc./Em.: 385/480 nm).
Run sphingosine standards (1-100 nmol) with unknown sphingolipid samples (from hydrolysis to fluorometry).
![]()
2 – Separation of the various classes of long-chain bases
Long-chain bases are separated according to the number of hydroxy groups present in the molecule.
The components may be derivatized (dinitrophenyl derivatives are frequently prepared) but the use of free bases can also be separated by TLC with good resolution.
Procedure:
The extract is chromatographed on silica gel G60 plates with chloroform/methanol/2M NH4OH (40/10/1, v/v).
Compounds are detected in UV light after primulin spray.
The detected spots are compared with the three most frequent long-chain bases, sphingosine, dihydrosphingosine and phytosphingosine chromatographed in a parallel lane. Sphingosine has the higher Rf and phytosphingosine the lower.
Spots are scraped and lipids are eluted by two washings with chloroform/methanol (2/1, v/v).
![]()
3 – Separation and quantification of long-chain bases
GLC PROCEDURES
The various long-chain bases may be analyzed by GLC if they are converted into non-polar compounds. To get better results it is recommended to derivatize first the amine group (N-acetyl derivatives) and then the hydroxyl groups (O-TMS derivatives).
Materials and Reagents:
Gas chromatograph equipped with a SE-30column (or equivalent).
acetic anhydride, methanol, hexane and derivatization reagents such as SIL-A from Sigma or BSTFA-TMCS.
Procedure:
The solution of long-chain bases is evaporated. The N-acetyl derivatization is done by reacting the free bases with 1 ml acetic anhydride/methanol (1/4, v/v) at room temperature overnight.
After evaporation of the anhydride solution under an efficient fume hood, bases are reacted with SIL-A (100 µl, 15 min at 30°C) or BSTFA-TMCS (100 µl, 4-5 h at 20°C).
After evaporation of the reaction medium, derivatized lipids are dissolved in a small volume of hexane and analyzed by GLC.
Sphingoid bases, mainly sphingosine and sphinganine, are easily determined by HPLC using a precolumn orthophthalaldehyde (OPA) labeling (reaction scheme shown below) to obtain high fluorescent detectability.
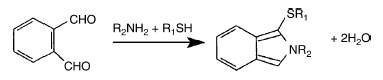
R2NH2 : fatty amine, R1SH : mercaptoethanol
We give below the optimized procedure by Yoon HT et al. (Arch Pharm Res 1999, 22, 294) which enables a quantitative estimation of sphingoid base in the range 20-800 pmol with a detection limit of 0.1 pmol as injected sphingosine amount.
Material and Reagents:
Standard sphingoid bases, 2-mercaptoethanol, OPA, ethanol, borate buffer pH 10.5, NH4OH, C18 reversed-phase column, fluorescent detector.
Procedure:
OPA reagent solution was prepared by dissolving 5 mg of OPA and 25 ml of 2-mercaptoethanol in 0.5 ml ethanol. The mixture is adjusted to 50 ml with 3% borate buffer (pH 10.5) and stored at 4°C (prepared weekly). A stock solution of sphingosine is prepared in dissolving 10 mg of base in 1 ml of ethanol with brief sonication.
The dry cellular lipid extract is dissolved in 40 ml of ethanol and preincubated at 60°C for 30 min. Then, 5 ml of OPA reagent are added to 40 ml of ethanol solution of lipids and incubated 30 min at room temperature.
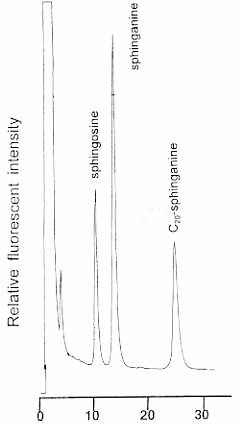
The OPA derivatives of sphingoid bases are separated by HPLC using 82% acetonitrile at a flow rate of 1 ml/min. The acetonitrile concentration is adjusted according to the HPLC column used. The derivatives are monitored fluorometrically at 340 nm for excitation and 455 for emission wavelength.
The alkaline digestion of acylglycerols enables to remove interferences in chromatographic elution and shortens the analysis time.
Precise quantitative results may be obtained using C20-sphinganine as an internal standard.
A modification was proposed using derivatization of sphingosine with naphthalene-2,3-dicarboxyaldehyde (He X et al., Anal Biochem 2005, 340, 113). That procedure was also used for the quantitative analysis of ceramide after hydrolysis with ceramidase. The limits of detection for derivatized sphingosine were about 40 fmol.
Separation of sphingosine phosphate
A HPLC method similar to that described above was adapted also for the determination of phosphorylated sphingoid bases (sphingosine 1-phosphate) in biological samples (Min JK et al., Anal Biochem 2002, 303, 167). The method consists of an enzymatic treatment to remove the phosphate group (if present) followed by the HPLC analysis of o-phthalaldehyde derivatives of the free bases. Levels of sphingoid bases from 0.5 to 100 pmol could be determined in several biological samples.
Other methods including different extraction, direct OPA derivatization procedures and HPLC separations have been described (Caligan TB et al., Anal Biochem 2000, 281, 36; Butter JJ et al., J Chromatogr B 2005, 824, 64).
Another HPLC-based approach was described (Andreani P et al., Anal Biochem 2006, 358, 239). That method was based on a simplified and efficient extraction method and a fluorescence labeling with 9-fluorenylmethyl chloroformiate (FMOC-Cl) instead of OPA. That procedure increased the sensitivity and lowered the detection limit of sphingosine and sphingosine-phosphate to 20 fmol.
A highly selective and sensitive technique based on liquid chromatography coupled with tandem mass spectrometry capable of reliable detection of less than 50 fmol of sphingosine-1-phosphate and dihydrosphingosine-1-phosphate was developed (Berdyshev EV et al., Anal Biochem 2005, 339, 129). Following synthesis of the bisacetylated derivatives of the sphingoid base-1-phosphates, the derivatives were subjected to negative ion spectrometry and linearly detected over wide ranges (0-300 pmol). The levels of the two sphingosine phosphates were determined in human plasma, fetal bovine serum, and human endothelial cells in culture.
DISPERSIVE LIQUID-LIQUID MICROEXTRACTION
Lire la suiteDevenez membre et participez au développement de la Lipidomique au XXIème siècle.
S'inscrire