
HPLC separation of lipid classes
With the previously described techniques, the quantification of the separated lipid classes represent a serious drawback since each fraction may need a separate treatment. Considerable progress has been made to, simultaneously, separate by HPLC and quantify with an efficient and near universal detector, the evaporative light-scattering detector (LSD).
The separation of the various simple and complex lipids present in natural extracts is easily managed through an isocratic or better with a gradient elution procedure. The non-specific LSD enables the quantification of non-polar and polar lipids in the same run.
It is recommended to first separate the crude lipid extract in two or three fractions by low pressure chromatography and analyze the fractions by HPLC . Thus, lower complex gradients and analysis time will be required.
Among several published procedures devoted to the separation of all lipid classes in one run, we have selected a simple and efficient procedure initially applied to the separation of lipid classes from plant lipids (Christie WW et al, J High Resol Chromatogr 1995, 18, 97).
Apparatus:
column: YMC PVA-Sil (250 x 4.6 mm, 3 µm from Hichrom), the phase is prepared by bonding a layer of polymerized vinyl alcohol to silica.
Ternary HPLC pump
Evaporative light-scattering detector
Reagents:
solvent A: isooctane/methyl tert-butyl ether (98/2, v/v)
solvent B: isopropanol/acetonitrile/chloroform/acetic acid (84/8/8/0.025, v/v)
solvent C: isopropanol/water/triethylamine (50/50/0.2, v/v)
The author has replaced isooctane by isohexane for safety reasons but with similar results.
Procedure:
A ternary gradient was generated during 40 min with a flow rate of 1 ml/min followed by a 10 min reequilibration time.
The optimum gradient is described below.
| Time (min) |
A |
B |
C |
Flow rate (ml/min) |
| 0 |
100 |
0 |
0 |
1 |
| 5 |
80 |
20 |
0 |
1 |
| 15 |
44 |
52 |
4 |
1 |
| 40 |
34 |
52 |
14 |
1.4 |
| 40.1 |
30 |
70 |
0 |
1.4 |
| 45 |
100 |
0 |
0 |
2 |
| 50 |
100 |
0 |
0 |
2 |
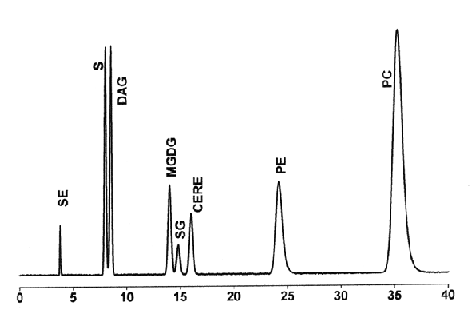
SE: sterol esters, S: sterols, DAG: diacylglycerols, MGDG: monogalactosyldiglycerides, SG: sterol glycosides, CERE: cerebrosides, DGDG: digalactosyldiglycerides, PE: phosphatidylethanolamine, PI: phosphatidylinositol, PC: phosphatidylcholine
The quantification of lipid compounds is made using appropriate standards, the relationships between sample size and response being dependent of the instrument used and the concentration ranges.
An HPLC method with LSD detection was optimized and validated for the simultaneous quantitation of cholesteryl esters, triglycerides, cholesterol and phosphatidylcholine in human plasma. A silica Spherisorb column was used with a multistep gradient system. The calibrations were made at levels of 0.14-14 mg lipid/injection (Seppanen-Laakso T et al., J chromatogr B 2001, 754, 437).
A reliable method was established to evaluate the lipid composition of plants. The procedure focused on the polar lipid distribution of rapeseed oil but was also applied to the estimation of waxes, triacylglycerols, and sterols (Beermann C et al., JAOCS 2003, 80, 747). The eluent system was modified from the method described above and the water eluent was supplemented with 1 mM ammonium sulfate to improve reproducibility. The gradient system was adapted to be suitable for the separation of major lipid classes of plant materials. A precise quantification was made on about 30 mg of total lipids using an evaporative light scattering detector.
Devenez membre et participez au développement de la Lipidomique au XXIème siècle.
S'inscrire