
This simplest polyol-phospholipid was first isolated in 1958 (Benson et al., Biochim Biophys Acta 1958, 27, 189) who described its structure. It can be defined as 1,2-diacyl-sn-glycero-3-phospho-1′-sn-glycerol.
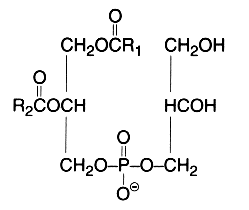
This lipid occurs widely but very low amounts are found in animal tissues (mainly in mitochondria), in plants it forms 20 to 30 % of total phospholipids (mainly in the chloroplast). In bacteria, trace amounts up to 70% of the total lipids are found. A dialkyl analogue forms a small proportion (about 5%) of the phospholipids from halophilic bacteria but a great proportion of the archaeal virus membranes (Vitale R et al., Biochim Biophys Acta 2013, 1831, 872). Its acidic properties require the use of acid mixtures for the extraction to prevent the tight association of the lipid with cations in the cell. Phosphatidylglycerol is the second most abundant phospholipid in lung surfactant since it is present at 7–15% of the total phospholipid. Its precise biological role in lung is unknown but it may play a role in alveolar stability and recent studies suggest that it regulates the innate immune response (Numata M et al., Proc Natl Acad Sci USA 2010, 107, 320).
An acylated form (the fatty acid being linked to the second glycerol) has been described, acyl phosphatidylglycerol, which is found in bacteria (Corynebacterium amycolatum) as a minor component (Yagüe G et al., FEMS Microbiol. Lett. 1997, 151, 125). The acyl group on the glycerol was only octadecenoyl acid. Acyl phosphatidylglycerol can be considered as a useful chemical marker for the identification of C. amycolatum in addition to the absence of mycolic acids. That phospholipid was also described in lipid extracts from a vegetal (oat, Avena sativa) where a high content of N-acylphosphatidylethanolamine was also observed (Holmback J et al., Lipids 2001, 36, 153).
A phosphorylated form (at the 3-position of the second glycerol), known as phosphatidyl glycerophosphate is found in halophilic bacteria (and in archaeal viruses) but is also an intermediate in the biosynthesis of diphosphatidylglycerol.
The existence of lysyl-phosphatidylglycerol was discovered in 1965 (Gale EF et al., Biochem J 1965, 94, 390)) and its metabolism described in 1971 in Staphylococcus aureus (Short SA et al., J Bacteriol 1971, 108, 219). Later, this compound was described in polar lipids of group B Streptococci (Fischer W, Biochim Biophys Acta 1977, 487, 89), in Caulobacter crescentus (Jones DE et al., Can J biochem 1979, 57, 424), in Bacillus subtilis (Deutsch RM et al., J Biol Chem. 1980, 255, 1521), and was shown to be a major component in Staphylococcus aureus and S. intermedius (Nahaie MR et al., J Gen Microbiol 1984 130, 2427). It was also detected in Vagococcus fluvialis (Fisher W et al., J Bacteriol 1998, 180, 2950), in several species of Listeria (Fisher W et al., Int J Syst Bacteriol 1999, 49, 653) and in Clostridium novyi (Guan Z et al., Biochim Biophys Acta 2011, 186-193). Thus, lysyl-phosphatidylglycerol is now a well-known membrane lipid in several gram-positive bacteria but is almost unheard of in gram-negative bacteria. It has been suggested that this phospholipid derivative may selectively protect bacteria against antimicrobial polypeptides (Ganz T, J Exp Med 2001, 193, F31).
An alanyl derivative of phosphatidylglycerol has been first discovered in Clostridium welchii (Macfarlane MG, Nature 1962, 196, 136), in C. novyi (Guan Z et al., Biochim Biophys Acta 2011, 186-193) and in many gram-positive bacteria (O’Leary WM et al., In “Microbial Lipids”. C. Ratledge et al., ed. Vol. 1. Acad Press, New York, NY. pp.117–201). Presumably, isomers carry the aminoacyl residue at different positions (O-1 or O-2) of the glycerol moiety.
Devenez membre et participez au développement de la Lipidomique au XXIème siècle.
S'inscrire