
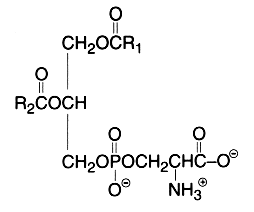
The main component of this group is a diacyl form: 1,2-diacyl-sn-glycero-3-phospho-L-serine or phosphatidylserine. It is the only amino acid-containing glycerophospholipid in animal cells.
Folch isolated a preparation of phosphatidylserine about 92% pure from brain “cephalin” by means of solvent fractionation and determined its exact composition (J Biol Chem 1948, 174, 439). This phospholipid occurs quite widely in nature but usually in concentrations less than 10% of the cell phospholipid pool. In brain tissue, myelin (white matter) has the highest amounts. Phosphatidylserine has generally highly unsaturated acyl chains.
From the formula it appears clearly that phosphatidylserine possesses three ionizable groups: a diester phosphoric acid, an amino group and a carboxyl function. Thus at pH 7, the phosphate and the carboxyl functions are in anionic form and the amino group is positively charged. When isolated, this lipid contains 1 mol of a cation (K or Na) which can be removed by washing the solvent with 0.05 N HCl.
Phosphatidylserine was not shown to be involved in cell signaling through the formation of metabolites (as phosphatidylcholine or phosphatidylinositol) but is a key component in the activation of various kinases (kinase C, Raf-1 kinase) as well as the blood coagulation process. Thus, externalization of phosphatidylserine from the inner to the outer membrane leaflet of platelets allows coagulation factors to bind and form enzyme/cofactor complexes and aids complement binding (Heemskerk JW et al., Thromb Haemost 2002, 88, 186). Loss of this ability to externalize phosphatidylserine (and phosphatidylethanolamine) is characteristic of the rare bleeding disorder Scott syndrome (Zwaal RF et al., Cell Mol Life Sci 2005, 62, 971).
For this last effect, it has been shown that both the PS head-group per se and unsaturation of the 1,2 fatty acids are important (Smirnov MD et al., Biochemistry 1999, 38, 3591). Later, two molecular species (18:0/20:4 and 18:0/18:1) have been shown to be the molecular determinants that regulate coagulation (Clark SR et al., PNAS 2013, 110, 5875).
However, lyso derivatives of phosphatidylserine were detected after corneal injury and may be involved in maintaining the integrity of the normal cornea in promoting cellular regeneration (Liliom K et al., Am J Physiol 1998, 274, C1065).
Phospatidylserine with ether linkage (1-0-alkyl glycerophosphoserines) were identified in the human lens (Deeley JM et al., Anal Chem 2009, 81, 1920). This is the first report of these phospholipids in human tissue.
Serine plasmalogens have been described in marine bivalves (Kraffe E et al., Lipids 2004, 39, 59). They were found to be enriched with non-methylene-interrupted fatty acids (mainly 7,17-22:2).
Phosphatidylserine is located entirely on the inner layer of the plasma membrane. This normal distribution is altered during platelet activation and cellular apoptosis. Thus, it was first demonstrated that red blood cells that are heavily loaded with that phospholipid are recognized and engulfed by macrophages (Tanaka Y et al., J Biol Chem 1983, 258, 11335). Later, it has been shown that phosphatidylserine is exposed on the surface of apoptotic cells (Fadok VA et al., J Immunol 1992, 148, 2207) and on the nuclei expelled from erythroid precursor cells (Yoshida H et al., Nature 2005, 437, 754). This signal works as an “eat me” signal for phagocytes. Further investigations have shown that a transmembrane protein (Tim4) is the phosphatidylserine receptor for the engulfment of apoptotic cells (Miyanishi M et al., Nature 2007, 450, 435). The phosphatidylserine exposure and its internalization are mediated by phospholipid scramblases and flippases, respectively. Both have recently been molecularly identified, and their functional mechanism and physiological roles are being elucidated (Nagata S et al., Curr Opin Immunol 2020, 62, 31).
A growing body of evidence indicates the involvement in apoptotic cells of oxidized phosphatidylserine as pattern recognition ligands for scavenger CD-36 receptors on macrophages (Greenberg ME et al., JEM 2006, 203, 2613).
The regulation of a pro-apoptotic factor, caspase-3, by phosphatidylserine rich in DHA was demonstrated in a model of neuronal cells ( Akbar M et al., Proc Natl Acad Sci 2005, 102, 10858). This mechanism may contribute to neurological deficits associated with n-3 fatty acid deficiency and support protective effects of DHA in pathological models such as brain ischemia or Alzheimer’s disease.
Phosphatidylserine was shown to modulate the activity of several key enzymes involved in cellular signaling. The detection of the movement of phosphatidylserine across membranes may be visualized using a complex of annexin V fused with a green fluorescence protein (Calderon F et al., J Neurochem 2008, 104, 1271).
The formation and the multiple functions of phosphatidylserine in mammalian cells have been reviewed (Vance JE et al., Biochim Biophys Acta 2013, 1831, 543).
A N-acylphosphatidylserine has been described in lipid extracts of mouse brain (Guan Z et al., Biochemistry 2007, 46, 14500). The complexity of this phospholipid is further increased by the presence of diverse amide-linked N-acyl chains, which include saturated, monounsaturated, and polyunsaturated species. N-Acylphosphatidylserine was also detected in the lipids of pig brain, mouse macrophage tumor cells, and yeast. It may be a biosynthetic precursor of N-acylserine which may have mediator functions.
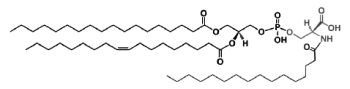
N-Acylphosphatidylserine
A graphical chart of the metabolism of phosphatidylserine may be found on a specific web site.
A derivative of phosphatidylserine, a phosphatidylserylglutamate, was isolated from Escherichia coli (Garrett TA et al., J Lipid Res 2009, 50, 1589). This minor lipid component has a polar head group formed by a serylglutamate residue. Several species with various fatty acids were discovered, the main one being R1 = 16:0 and R2 = 18:1n9.
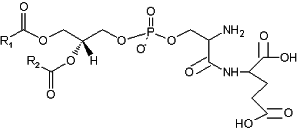
Phosphatidylserylglutamate
Lysophosphatidylserine has long been known as a signaling lipid in mast cell biology, markedly enhancing histamine release (Smith GA et al., FEBS Lett 1979, 105, 58) and eicosanoid production. More recently, there has been a resurgence of interest in lysophosphatidylserine as new roles in the promotion of phagocytosis of apoptotic cells have been studied. An important review of the actually known roles of lysophosphatidylserine may be consulted (Frasch SC et al., Prog Lipid Res 2012, 51, 199).
Devenez membre et participez au développement de la Lipidomique au XXIème siècle.
S'inscrire