
The nature of these compounds was discovered during the 19th century. In 1831, Wachenroder H. proposed the term “carotene” for the hydrocarbon pigment he had cristallized from carrot roots. J Berzelius called the more polar yellow pigments extracted from autumn leaves “xanthophylls” and M Tswett, who separated many pigments by column chromatography, called the whole group “carotenoids“.
Information on their physical and chemical properties as well as spectroscopic and chromatographic data for the determination of carotenoids in biological samples has been given (Rivera Vélez SM, J Nat Prod 2016, 79, 1473).
Among this important group, the numerous compounds consist of C40 chains (tetraterpenes) with conjugated double bonds, they show strong light absorption and often are brightly colored (red, orange). These pigments are widely distributed as naturally occurring constituents of fruits and vegetables, bacteria and algae. They cause the coloration of a wide variety of flowers, birds and marine animals. They are often added to human foodstuffs in order to achieve acceptable food coloration as they strongly absorb light in the region of 400–500 nm and are colored red, orange, and yellow.
The great interest in the production of carotenoids on a large scale has urged the development of approaches for their rapid extraction and purification from marine algae with green solvents. Among all the possible techniques, those based on aqueous media, applicable to fresh biomass, have been proposed, The use of aqueous solutions of surfactants seems very efficient and easy to adapt to industrial applications (Vieira FA et al., Mar Drugs 2019, 17, 310).
Carotenoids perform three major functions in plants : accessory pigments for light harvesting, prevention of photo-oxidative damage and pigmentation attracting insects.
Carotenes contain only carbon and hydrogen, i.e., they are true hydrocarbons. Prominent members include α-carotene, β-carotene, and lycopene. Carotenoids containing oxygen include lutein and zeaxanthin. They are known as xanthophylls. Carotenes and xanthophylls are, by far, the predominant class of tetraterpenes. They may also be classified in the terpenoids.
To date, more than 1200 carotenoids (and apocarotenoids) have been identified from more than 700 producing organisms. The Japanese Carotenoid Database (http://carotenoiddb.jp/ (accessed in September 2022)) is the most comprehensive and detailed database to find updated information regarding structural diversity, biological functions, producing organisms, and classification of carotenoids.
Only 50 carotenoids are regularly consumed in the human diet and 24 have so far been detected in human plasma. In Western diets, the most abundant carotenoids are the three oxygenated xanthophylls lutein, zeaxanthin and β-cryptoxanthin and the three major carotenes, α-carotene, β-carotene and lycopene. The human intake of carotenoids may be appreciated using databases such as that established for Swiss vegetables (Reif C et al., J Food Comp Anal 2013, 29, 64).
There are C30 (composed of 30 carbon atoms), C40, C45 and C50 carotenoids, distributed in all 3 kingdoms of life (eukaryotes, prokaryotes and archaea. About 800 carotenoids have been described in eukaryotes and only 25 carotenoids have been detected in Archaea (including 13 exclusively found in Archaea).
Carotenoids may be acyclic (seco-carotenoids) or cyclic (mono- or bi-, alicyclic or aryl). As both are carotenoids, xanthophylls and carotenes are similar in structure, but the oxyfunctionalization of various carotenoids leads to a large number of xanthophylls in which the function may be a hydroxyl, methoxyl, carbonyl, oxo, formyl or epoxy group. Their high structural diversity is based on the possibilities of cyclization of terminal carbons, substitution pattern and chemical modification (hydroxylation, epoxidation, carboxylation, carbonylation, glycosylation, unsaturation, alkoxylation, isoprenic polymerization, lactonization, sulfation, cycloaddition, etc.) and isomerization (cis/trans isomerization).
Illustration of the structural diversity of carotenoids. Possible terminal groups by cyclization and structural modifications identified in natural carotenoids. From http://carotenoiddb.jp/
In microorganisms, carotenoids play a key role in the processes of photoprotection, photosynthesis, phototropism and resistance to oxidative stress. Some carotenoids have been described as virulence factors in bacteria, permitting the cells to fight against the oxidative stress caused by phagocytes (e.g., staphyloxanthin, a carotenoid antenna of xanthorhodopsin, produced in Staphylococcus aureus).
Importantly, some carotenoids, such as salinixanthin or thermozeaxanthin, are only produced by extremophilic microorganisms, suggesting a potential function in thermal, pH or salinity adaptation (Mandelli F et al., World J Microbiol Biotechnol 2012, 28:1781 ; Jehlička J et al., Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 106, 99) (review in : Grivard A et al., Mar. Drugs 2022, 20, 524).
Carotenoids are important components of the light harvesting in plants, expanding the absorption spectra of photosynthesis. They are universally found in green tissues, located in the chloroplast thylakoid membranes, associated with the reaction centers and antenna complexes of photosystems I and II. They are in pigment‐protein complexes to ensure the right positioning and orientation essential for efficient energy transfer. They also protect plant tissues by helping to absorb the energy from singlet oxygen, an excited form of the oxygen molecule O2 which is formed during photosynthesis. The major carotenoids in this context are lutein, violaxanthin and neoxanthin.
In mammals, carotenoids exhibit immunomodulatory actions, likely related to their anticarcinogenic effects. β–Carotene was thus shown to enhance cell-mediated immune responses (Hughes DA, Proc Nutr Soc 1999, 58, 713). The decrease in prostate cancer risk has been linked to the consumption of tomatoes, vegetable rich in lycopene, as prostatic tissues. While there is yet limited direct evidence linking lycopene and prostate cancer (Kavanagh CJ et al., J Natl Cancer Inst 2007, 99, 1074), several observations, including the ability of the prostate to concentrate lycopene, suggest a special protection of lycopene against that pathology (Stanley JC, Lipid technol 2008, 20, 64 ; Moran SE et al., J Nutr 2022, 152, 1381).
Animals are unable to synthesize carotenoids de novo so they are obtained from the diet. Ingested carotenoids are either accumulated unchanged or are slightly modified into typical animal carotenoids. A comprehensive treatise dedicated specifically to the multiple facets of food carotenoids has been published (Food carotenoids – chemistry, biology, and technology, DB Rodriguez-Amaya, IFT Press, Wiley Blackwell, 2016).
A comprehensive review on the chemistry of carotenoids and their physiological effects on human health may be found (Langi P. et al., Methods Mol Biol. 2018, 1852, 57). A review of marine microalgae indicate that numerous carotenoids exhibit direct antimelanoma effect but are also capable of restoring melanoma cells sensitivity to conventional chemotherapy (Ferraz C et al., Marine Drugs 2022, 20, 618). The potential use of marine archaea, bacteria, algae, and yeast as biological factories of carotenoids has been reviewed (Mapelli-Brahm P et al., Mar Drugs 2023, 21, 340). Some bactericidal aspects of carotenoids have been shown in vitro, a few studies have also demonstrated they have a prebiotic-like effect. They may further contribute to mucosal and gut barrier health, such as stabilizing tight junctions. Potential gut-related health-beneficial effects of carotenoids regarding carotenoid—gut microbiota interactions have been reviewed (Eroglu A et al., Adv Notre 2023, 14, 238). A review may be consulted on the biological activities of key carotenoids (lycopene, lutein, astaxanthin, β-carotene, fucoxanthin, and canthaxanthin), their structures, sources, market, economical features, and stability (Gholipour-Varnami K et al., Food Biosci 2025, 68, 106529).
Carotenoids consist of eight isoprenoid units joined in such a manner that the arrangement of isoprenoid units is reversed at the center of the molecule so that the two central methyl groups are in a 1,6-position relationship and the remaining non-terminal methyl groups are in a 1,5-position relationship. They are, by far the predominant class of tetraterpenes. They may be also classified in the terpenoids.
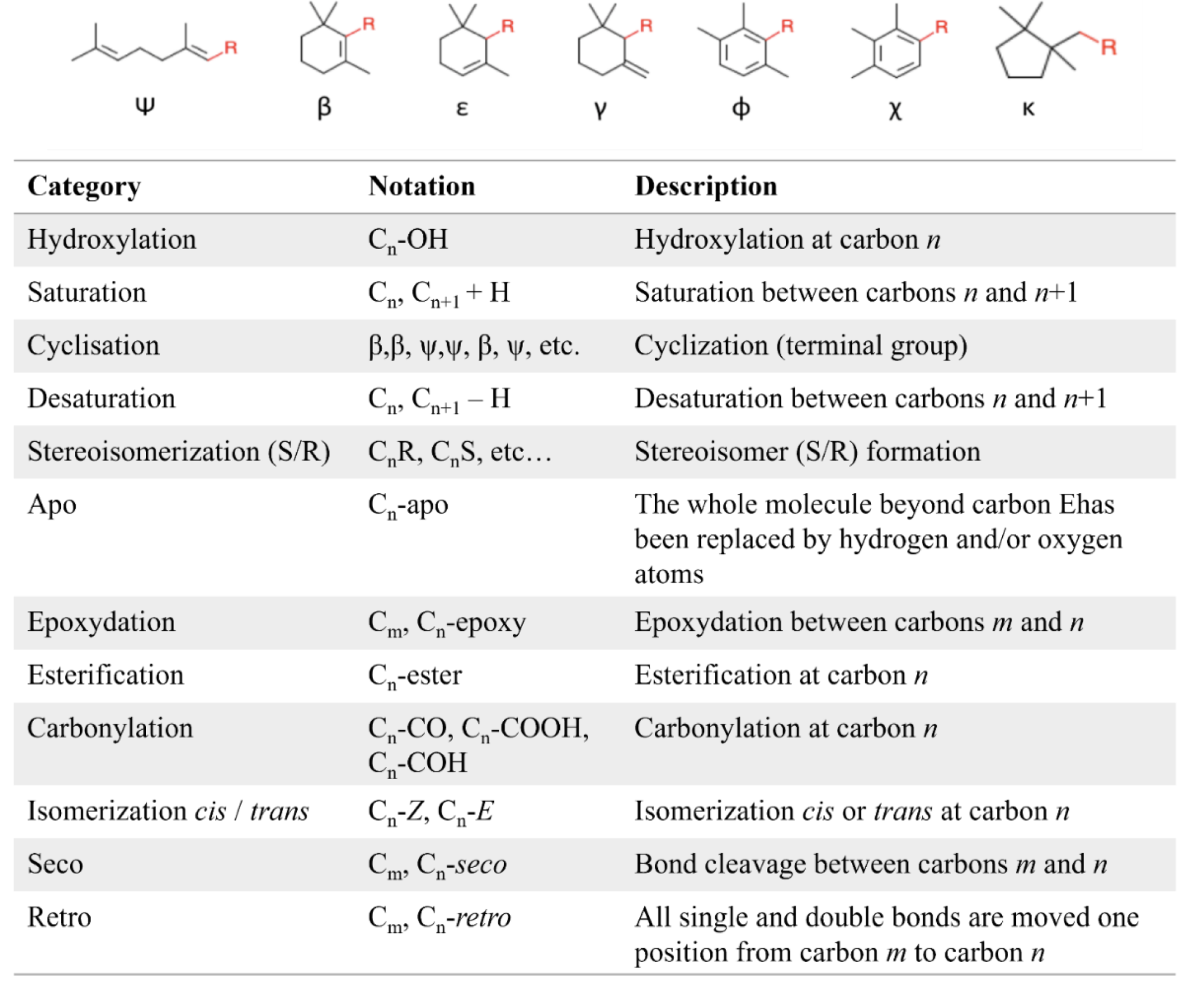
Carotenes
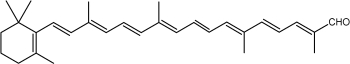
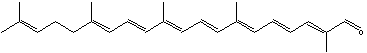
Carotenes may be considered as derivatives of lycopene, found in tomatoes, fruits and flowers. Its long straight chain is highly unsaturated and composed of two identical units joined by a double bond between carbon 15 and 15′. Each of these 20 carbon units may be considered to be derived from 4 isoprene units. Lycopene is a bioactive red colored pigment naturally occurring in plants. Interest in lycopene is increasing due to increasing evidence proving its antioxidant activities and its preventive properties toward numerous diseases. In vitro, in vivo and ex vivo studies have demonstrated that lycopene-rich foods are inversely associated to diseases such as cancers, cardiovascular diseases, diabetes, and others. A review of all these aspects may be consulted (Kong KW et al., Molecules 2010, 15, 959). Lycopene was used in human as a supplement alone or in combination with other foods and was shown to be useful for developing treatments against liver disease with reduced contraindications (Donghia R et al., Nutrients 2024, 16(4), 562).
Only some of the most common carotenes are given below:
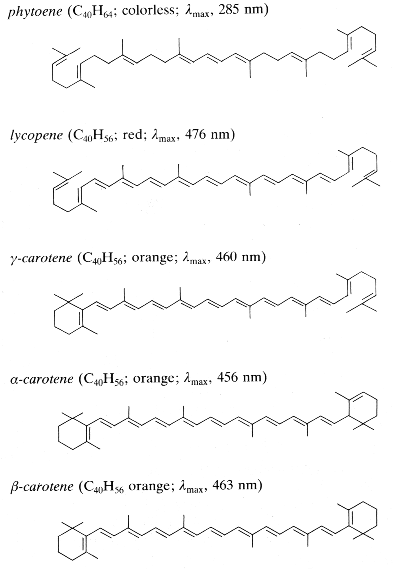
Phytoene is one of the first intermediates in the biosynthesis of carotenoids. It is formed by coupling of two molecules of geranylgeranyl pyrophosphate by the action of the phytoene synthase. The diphosphate is removed and proton shift leads to the formation of phytoene. It is a colorless product and absorbs light in the UV range only. Dietary phytoene is accumulated in human skin where it can potentially protect the skin (UV absorber, antioxidant, anti inflammatory) (Aust O et al., Int J vitam Nutr Res 2005, 75, 54).
Phytofluene, another colorless carotenoid, has 10 double bonds, but only 5 conjugated double bonds, much lower as compared to coloured carotenoids (they must have at least 7 of these bonds to be coloured. Thus, unlike coloured carotenoids, phytoene and phytofluene absorb maximally in the ultraviolet region (286 and 348 nm, respectively).
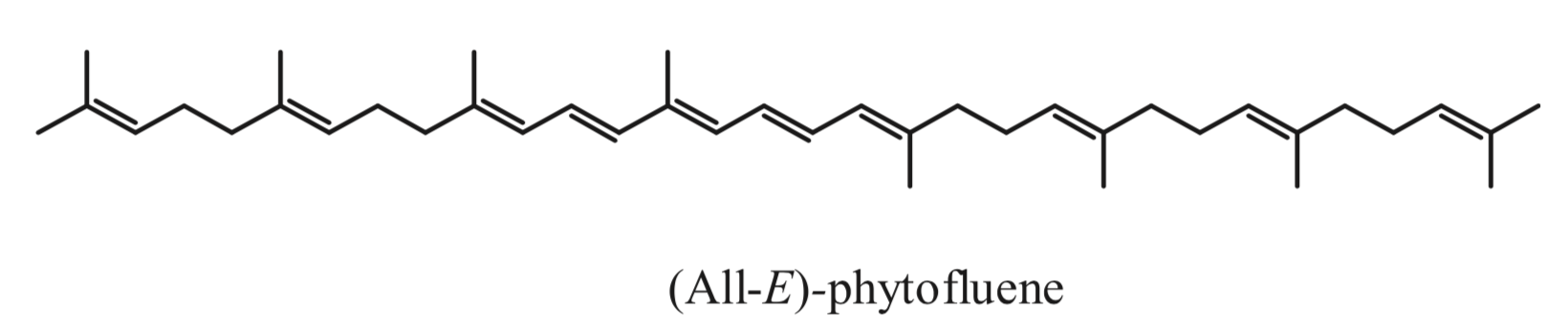
Despite being rarities among carotenoids, phytoene and phytofluene are very important for a series of reasons and are attracting much interest recently from diverse point of views. A number of studies indicate that they may be related to the reduction of the risk of developing certain cancers, such as skin, prostate, leukemia, breast and endometrial cancer, and atherosclerosis. A review has summarized much of the research carried out regarding these carotenoids in the context of food science, nutrition and health; highlighting their potential in the context of functional foods and nutricosmetics (Meléndez-Martínez AJ et al., J Food Comp Anal 2018, 75, 91-103).
Among the represented molecules, β-carotene is probably the most important as a precursor of vitamin A by central cleavage into retinol and some retinoic acid (β-carotene provides about 40% of human dietary retinol equivalents).
It has been observed that serum α-carotene, but not other antioxidants, was positively associated with muscle strength in older adults (Bruno RR et al., Antioxidants 2022, 11, 2386). In that survey, 1172 individuals, aged between 50 and 85 years, α-carotene levels were positively associated with muscle strength evaluated by the isokinetic knee extension test. Clearly, it is not possible to establish causality, since this is cross-sectional study, further clinical trials being necessary to evaluate the effect of the increased intake of α-carotene food sources on muscle strength in older adults. In a large cross-sectional study of about 2000 older adults followed during 3 years, it was determined that higher dietary α-carotene and β-carotene intake has inverse effects on cognitive function decline (Zhong Q et al., Nutrients 2023, 15, 239). A large investigation has demonstrated that Increased dietary intakes of various carotenoids were associated with lower biological aging indices, which was possibly and mainly driven by lutein/zeaxanthin and β-carotene (Qi X et al., Nutr J 2025, 24, 9). It has been observed that increasing levels of blood carotenoid were significantly associated with longer leukocyte telomeres in US adults. High intake of carotenoid-rich food may play a role in protecting telomeres and regulating telomere length, possibly associated with health- and aging-related diseases caused by oxidative stress (Min KB et al., Eur J Nutr 2017, 56, 1045).
Carotene and lycopene are known to improve skin properties when ingested as supplements or in vegetal products.They protect against sunburn, increasing the basal defense against UV light-mediated damage to the skin, although their efficacy is not comparable to the use of a sunscreen (Aust O et al., Int. J Vitam Nutr Res 2005, 75, 54–60). β-Carotene has been used for several years to ameliorate the skin photosensitivity arising from excess protoporphyrin (Mathews-Roth MM et al., Arch Dermatol 1977, 113, 1229). The link between the clinical, in vivo and ex vivo results with the underlying molecular mechanisms with an emphasis on antioxidative processes has been reviewed (Black HS et al., Antioxidants. 2020, 9, 264).
The majority of carotenoids are still manufactured chemically, but, currently, the microbial production of β-carotene is of commercial importance. Blakeslea trispora is one of the best fungal strain employed for the production of β-carotene, commercially utilized by DSM. B. trispora produces up to 3% carotene per cell dry weight. B. trispora is also employed for the production of natural lycopene, commercialized by Vitatene. The marine algae Dunaliella salina is also employed for manufacturing of β-carotene (Cognis, Proalgen Biotech Ltd.).
β-Carotane, the fully hydrogenated derivative of β-carotene (or fully saturated), is frequently found in sediments, associated with anoxic, saline lacustrine or some marine settings where organisms such as unicellular algae Dunalliela bloom and thrive as the dominant biota.
Torulene (3′,4′-didehydro-β,γ-carotene) is a carotene which is notable for being synthesized by red pea aphids (Acyrthosiphon pisum). It has been been demonstrated that these insects have gained the ability to synthesize torulene by horizontal gene transfer of a number of genes for carotenoid synthesis, apparently from fungi (Takema F et al., Science. 2010, 328 (5978), 574). It appears that torulene is the only carotenoid known to be synthesized by an organism different from plants and microorganisms. This red carotenoid, produced basically by Rhodotorula species, has gained significant attention for its red color and potential health benefits. A study has contributed to the understanding of torulene’s versatility and potential market applications (Mussagy CU et al., Food Biosci 7 July 2025, 107201). Its microbial production and potential applications have been reviewed (Jin J et al., Food Sci Biotechnol 2024, 34(11), 2417).
 Torulene
Torulene
Specific carotenoids are found in mineral sediments or crude oils and are used as biomarkers. Among them, isorenieratane, found in oils of Devonian age, is derived from the carotenoid isorenieratene (an homologue of the previous one but with an unsaturated isoprenoid chain), which is synthesized by photosynthetic green sulfur bacteria (Chlorobiaceae)(Koopmans MP et al., Geochim Cosmochim Acta 1996, 60, 4467).
Isorenieratane
Another C40 carotenoid, paleorenieratane, also thought to be derived from the same bacteria has been identified in Devonian aged sediments and crude oils (Hartgers WA et al., Organic Geochem 1994, 22, 703).
Paleorenieratane
These green sulfur bacteria are strict anaerobes that require light and hydrogen sulfide in stratified water columns to carry out photosynthesis and are thus markers for these photic zones (euxinic) in depositional environments.
Carotene and lycopene are known to improve skin properties when ingested as supplements or in vegetal products.They protect against sunburn, increasing the basal defense against UV light-mediated damage to the skin, although their efficacy is not comparable to the use of a sunscreen (Aust O et al., Int. J Vitam Nutr Res 2005, 75, 54–60). β-Carotene has been used for several years to ameliorate the skin photosensitivity arising from excess protoporphyrin (Mathews-Roth MM et al., Arch Dermatol 1977, 113, 1229). The link between the clinical, in vivo and ex vivo results with the underlying molecular mechanisms with an emphasis on anti-oxidative processes has been reviewed (Black HS et al., Antioxidants. 2020, 9, 264).
Xanthophylls
Only some of the most common xanthophylls are given below:
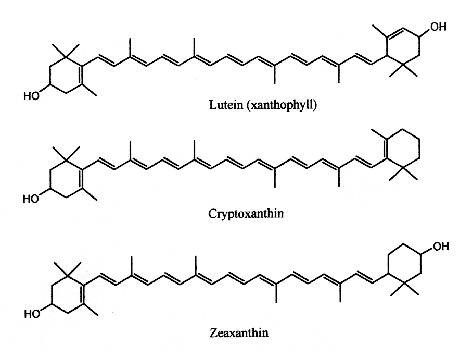
Xanthophylls
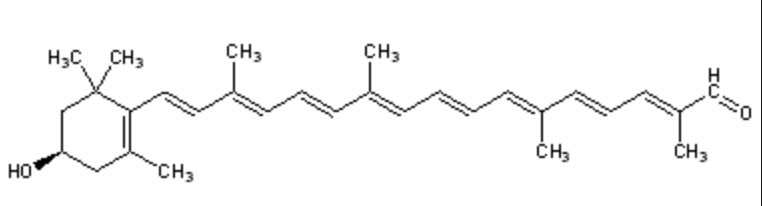
Lutein has its maximum absorption at 450 nm, cryptoxanthin at 453 nm and zeaxanthin at 454 nm. Egg yolk usually contains about 175-400 mg of lutein and about 200-300 g of zeaxanthin, but this could be affected by hen’s feed composition (Handelman GJ et al., Am J Clin Nutr 1999, 70, 247). Up to 18% losses of xanthophylls have been observed after various cooking methods (Nimalaratne C et al., J Agric Food Chem 2012, 60, 12547).
Cryptoxanthin is the main pigment of many orange‐fleshed fruits, such as peach, nectarine, orange‐fleshed papaya, persimmon, fruit of the tree tomato, and the Brazilian fruit Spondias lutea. This compound shows a positive correlation between the intake of β-cryptoxanthin and the prevention of several diseases. In fact, this molecule is characterized by having provitamin A activity, anti-obesity effects, antioxidant activities, and anti-inflammatory, and anti-tumor activity (Jiao Y et al., Curr. Pharmacol. Rep. 2019, 5, 20).
Lutein and zeaxanthin are found in human serum but are also present in retina, macula (the central region of the eye (they are frequently referred to as macular pigment), and lenses. Thus, they are at the origin of the name of the central part of the retina, macula lutea (yellow spot). By absorbing blue-light, the macular pigments protect the underlying photoreceptor cell layer from light damage, possibly initiated by the formation of reactive oxygen species. Increasing the intake of lutein or zeaxanthin may prove to be protective against the development of age-related macular degeneration (Krinsky NI et al., Annu Rev Nutr 2003, 23, 171). A systematic review and network meta-analysis of randomized controlled trials have been done on the effect of antioxidant supplementation on macular pigment optical density and visual functions (Hu W et al., Adv Nutr 2024, 15, 100216).
Furthermore, pro-vitamin A carotenoids such as α-carotene, β-carotene and β-cryptoxanthin may be important for neurocognitive development due to their ability to convert into vitamin A (Bohn T, In: Preedy VR (ed) Vitamin A and carotenoids: chemistry, analysis, function and effects. Royal Society of Chemistry, Cambridge, pp 142–161). Lutein and zeaxanthin in neural tissue may have biological effects that include antioxidation, anti-inflammation, and structural actions. In young people, the relative contribution of lutein to the total carotenoids in the brain is twice that found in adults. The greater proportion of lutein in the children brain suggests a need for lutein during neural development as well. In adults, higher lutein status is related to better cognitive performance, and lutein supplementation improves cognition (Johnson EJ, Nutr Rev 2014, 72, 605). The presence of lutein in breast milk, the preferential accumulation in the brain and evidences of influence on the retina and the functionality of the brain signal strongly emphasize the role of lutein and zeaxanthin on visual maturation and brain development (review in: Ramirez M, OCL 2016, 23/1, D107).
Investigators have found that there were no differences in serum lutein responses to free or ester form after regular ingestion of marigold lutein (Olmedilla-Alonso B et al., Nutrients 2024, 16, 1415). Importantly, daily supplementation with lutein for 15 days significantly increased serum lutein in normolipemic adults to levels associated with a reduced risk of age-related eye disease regardless of the chemical form of lutein supplied.
Many studies suggest that zeaxanthin has protective effects ofagainst chemical, natural, and radiation-induced toxicities (review in: Foroushan SK et al., J Funct Foods 2024, 121, 106436).
It has been also shown that lutein and zeaxanthin, but also β-cryptoxanthin and β-carotene present in brain regions of infants were associated with memory, executive function, vision, and hearing (Vishwanathan R et al., J Pediatr Gastroenterol Nutr 2014, 59, 659). New studies provided data strongly suggesting a potential role of prenatal carotenoids, particularly β-cryptoxanthin, on early offspring cognitive and motor development (Lai JS et al., Eur J Nutr 2021, 60, 703).
Studies exhibiting neuroprotective properties of lutein against neurodegenerative conditions, more specifically Altzeimer’s disease and Parkinson’s diseases in various model systems as well as clinical observations have been reviewed (Saisree Iyer et al., Mol Nutr Food Res 2024, 68, 2300409). Accordingly, the concerns associated with lutein absorption and potential strategies to improve its bioavailability have been discussed.
Saproxanthin is an uncommon and recently described natural CA found in algae, bacteria, and archaea (Novoveská L et al., Mar. Drugs 2019, 17, 640), bacteria being the main source.
Saproxanthin
This compound is a potent antioxidant. It is produced by algae with the aim to protect itself from the activated oxygen produced by light. In vitro studies have shown it has high antioxidant activity against lipid peroxidation in the rat brain homogenate model and a neuroprotective effect.
Myxol is a derivative of γ-carotene and is present in different forms in nature (free or combined with fucosides or nitrogen groups). Nevertheless, in the free state, it is found primarily in marine environments (Torregrosa-Crespo J et al., Mar. Drugs 2018, 16, 203).
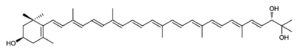 Myxol
Myxol
This molecule has been proved to have antioxidant properties. In fact, its antioxidant activity is greater than that of other frequently used antioxidant molecules such as zeaxanthin and β-carotene. One study was able to demonstrate significant antioxidant activities against lipid peroxidation in the rat brain homogenate model and a neuroprotective effect of l-glutamate toxicity. These findings indicate that the monocyclic structure of carotenoids exhibits various biological activities, including activities that provide health benefits.
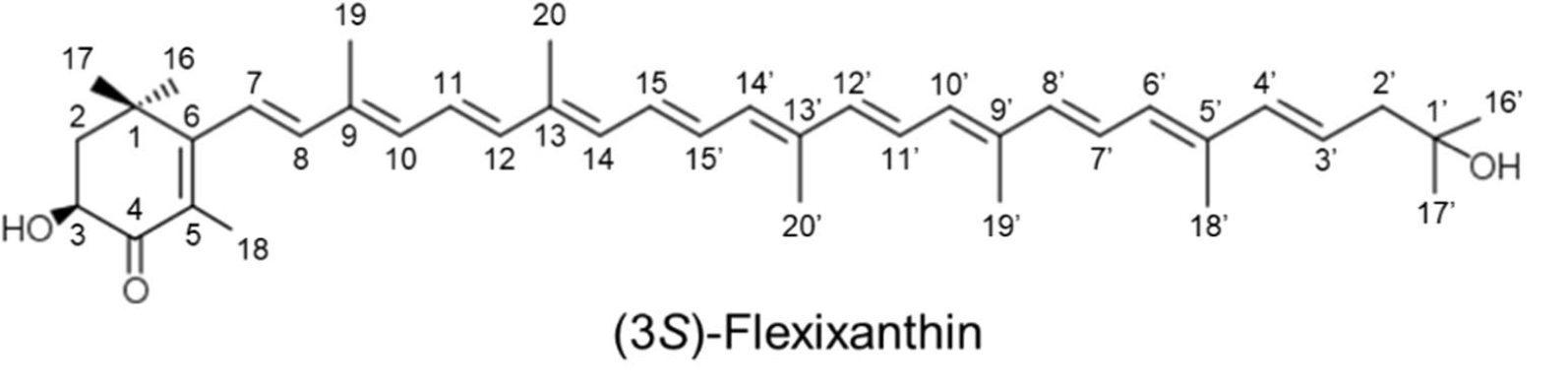
A new red-pigmented Algoriphagus sp. strain, oki45, was isolated from the surface of seaweed collected from Okinawa, Japan. Analyses revealed that the main carotenoid present in these bacteria was a mixtures of the monocyclic carotenoids, flexixanthin and 2-hydroxyflexixanthin being the most concentrated (Takatani N et al., Appl Microbiol Biotechnol 2024, 108, 102). The biological activities of flexixanthin and its derivatives were evaluated in terms of their antioxidant and anti-inflammatory effects against macrophages (Takatani N et al., Food Biosci 28 January 2025, 106015). These results indicate that the introduction of hydroxyl groups at specific sites affects the antioxidant and anti-inflammatory activities of flexixanthin.
Rubixanthin, a derivative of γ‐carotene, is the main pigment of rose hips and also occurs in appreciable amount in pitanga.
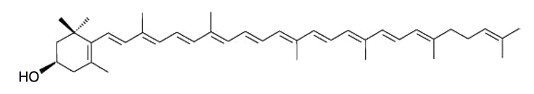 Rubixanthin
Rubixanthin
Xanthophylls in green leaves are unesterified, and those of corn are mostly unesterified. Xanthophylls in ripe fruits are generally esterified with fatty acids. However, the carotenols of a few fruits, particularly those that remain green when ripe, such as kiwi, undergo limited or no esterification.
Several nutritional investigations have suggested that lutein supplements may improve visual function and optical density in aged people (Olmedilla B et al., Nutrition 2003, 19, 21).
Natural aromatic derivative of lutein, 3,3′-dihydroxyisorenieratene and its non-hydroxylated parent compound, isorenieratene, are produced by green sulfur bacteria and byBrevibacterium linens, a bacteria used in the dairy industry for production of smear cheese (Saint Paulin, Münster, St Nectaire) (Rattray FP et al., J Dairy Sci 1999, 82, 891). Their high antioxidant activity against free radicals and their specific ability to prevent oxidatively generated damage to DNA make them interesting compounds for the development of endogenous photoprotective systems (Wagener S et al., Free Rad Biol Med 2012, 53, 457).
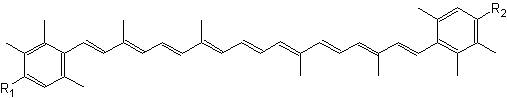
3,3′-Dihydroxyisorenieratene (R1, R2 = OH)
Isorenieratene (R1, R2 = H)
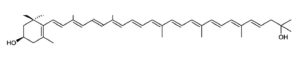
Astaxanthin, present in yeast, microalgae, crustaceans and fish, is a natural nutritional component, used as a food supplement for human and animal consumption. Its commercial production comes from both natural and synthetic sources.
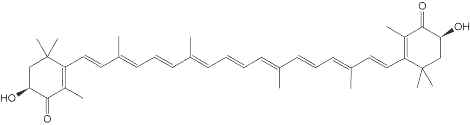
Astaxanthin
Astaxanthin can be synthesized only by some bacteria (such as Agrobacterium aurantiacum), fungi (such as Xanthophyllomyces dendrorhous), and microalgae (such as Haematococcus pluvialis). Haematococcus is considered the most promising source of natural astaxanthin at industrial scale. Animals obtain astaxanthin from their diets either by directly ingesting astaxanthin-rich prey or by converting other ingested carotenoids into astaxanthin. Haematococcus is considered the most promising source of natural astaxanthin at industrial scale.
Thus, astaxanthin can be naturally found in widely consumed seafood such as salmon and marine invertebrates such as krill, lobster, and shrimp. Astaxanthin is also found in birds like flamingo and quail. Free astaxanthin is particularly sensitive to oxidation. Consequently, it is usually found either conjugated to proteins, like in salmon muscle or lobster exoskeleton, or esterified with one or two fatty acids (monoester and diester forms), which stabilizes the molecule.
Astaxanthin shows very good capability at protecting membranous phospholipids and other lipids against peroxidation. Several studies have shown that astaxanthin has a stronger antioxidant activity than vitamin E (up to 500 times) and other carotenoids. In animal models, its modulates immune response (Bennedsen M et al., Immunol Lett 1999, 70, 185), inhibits cancer cell growth (Chew BP et al., Anticancer Res 1999, 19, 1849), and reduces bacterial load and gastric inflammation in vitro and rodent models. In humans, dietary astaxanthin decreases a DNA damage biomarker and acute phase protein, and enhances immune response (Park JS et al., Nutr Metabol 2010, 7, 18). Several research suggest that astaxanthin has protective effects against light-induced retinal damage via the mechanism of its antioxidative effect (Otsuka T. et al., J Pharmacol Sci. 2013, 123, 209). Furthermore, dietary astaxanthin accumulates in the skin and appears to prevent the effects of UVA irradiation on filaggrin metabolism and desquamation in the epidermis and the extracellular matrix in the dermis (Komatsu T. et al., PLoS One. 2017 Feb 7;12(2):e0171178). A study indicates that dietary astaxanthin accumulates in the skin and appears to prevent UV light-induced skin damage, and the Z-isomers are more potent oral sunscreen agents than the all-E-isomer (Honda M et al., Mar. Drugs 2022, 20(7), 414).
More importantly, astaxanthin has been observed to slow down brain aging by increasing brain-derived neurotrophic factor levels in the brain, attenuating oxidative damage to lipids, protein, and DNA and protecting mitochondrial functions. The scientific interest in the field of natural compounds with geroprotective activities is growing exponentially (Sorrenti V et al., Mar Drugs 2020, 18, 351). Emerging evidence suggested that biological activities for astaxanthin may counteract the pathophysiology features of parkinson disease, revealing a promising therapeutic potential in the prevention or onset of symptoms in PD patients (Castelli V et al., Aging 2020, 12, 4641). Several results indicated that a astaxanthin-enriched diet, attenuated the loss of dopaminergic neurons. A review offered a comprehensive and holistic approach to the analysis of the biopharmaceutical and nutraceutical potential of astaxanthin in the context of neurodegenerative diseases (Yordi EG et al., Marine Drugs 2024, 22(7), 327).
It was also demonstrated in vitro that astaxanthin has a potential ability to suppress the human prostate LNCaP cells growth at high concentrations. It was able to repress the testosterone-induced transcription of androgen resptor-target genes (Montazeri-Najafabady N et al., J Food Biochem 2021, 45/4, e13702). An in vivo study has investigated the inhibitory effect of astaxanthin on testosterone-induced benign prostatic hyperplasia in rats (Wang L et al., Marine Drugs 2021, 19, 652) demonstrating an important inhibitory effect, possibly due to superoxide dismutase activity regulation and testosterone and dihydrotestosterone levels. Astaxanthin (as fucoxanthin) shows antimicrobial activity, which was presented in a review article (Karpiński TM et al., Marine Drugs 2022, 20, 36).
Astaxanthin has two types of applications in animal husbandry: as a source of natural color and as a nutritional supplement because of its physiological effects (survivability, growth, photoprotection, fertility, egg quality, protection from cataracts). In Humans, users reported that astaxanthin was as effective as, or more effective, than anti-inflammatory drugs. A growing body of evidence suggests that astaxanthin is efficacious in the prevention and treatment of several ocular diseases. A review provides an evaluation of clinical applications of astaxanthin in the management of retinal diseases, ocular surface disorders, uveitis, cataract and asthenopia (Giannaccare G et al., Mar Drugs 2020, 18, 239). A review aims to explore the potential therapeutic use of astaxanthin in neurological and neuroinflammatory diseases (Bahbah EI et al., Mar. Drugs 2021, 19(4), 201). A comprehensive overview of the intricate relationship between aging, adult neurogenesis, and the potential neuroregenerative properties of astaxanthin, may be consulted (Medoro A et al., Mar. Drugs 2023, 21(12), 643). By modulating these pathways, along with its potent antioxidant properties, astaxanthin may contribute to the restoration of a healthy neurogenic microenvironment, thereby preserving the activity of neurogenic niches during both normal and pathological aging.
In the plant kingdom, Arabidopsis thaliana lines overexpressing the Chlamydomonas reinhardtii β-carotene ketolase accumulated high amounts of astaxanthin in the leaves was shown to have lower sensitivity to abscisic acid, to have increased tolerance to oxidative stress and to exhibit enhanced resistance to the bacterial pathogen Pseudomonas syringae pv. tomato DC3000 (Yu L et al., J Agric Food Chem 2019, 67, 12590). That approach provides insight into new roles of carotenoids in plant response to oxidative stress and pathogen infection.
A review discussed the significance of astaxanthin esters as functional food ingredients, highlighting their nutritional value, phytochemical structure, biological activities, and potential applications in the food industry (Wang Y et al., J Agric Food Chem 2024, 72, 13467).
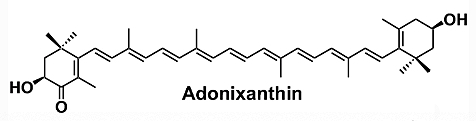
Adonixanthin is a microalgal astaxanthin derivative with a high potential in industry (Maoka T, J Nat Med. 2020, 74, 1). It is a unique keto-carotenoid containing two polar ionone rings. Compared to the astaxanthin structure, only one of the ionone rings carries a ketone group. This causes polarity and could result in stronger protective effects than astaxanthin (Iwata S et al., Brain Res. 2018, 1698, 130). Adonixanthin was reported to show very promising results in anti-tumor and anti-cancer treatment and protection against brain damage induced by cerebral hemorrhage (Maoka T et al., J Oleo Sci 2013, 62, 181).
Rhodoxanthin is a xanthophyll pigment with a purple color that is found in small quantities in several plants as Taxus baccata and Lonicera morrowii and in the feathers of some birds. Several isomers were predominant in the red-colored arils of Taxus (Schex R et al., Phytochemistry 2021, 186, 112741). It is used as a food additive in some countries (Australia, New Zealand).
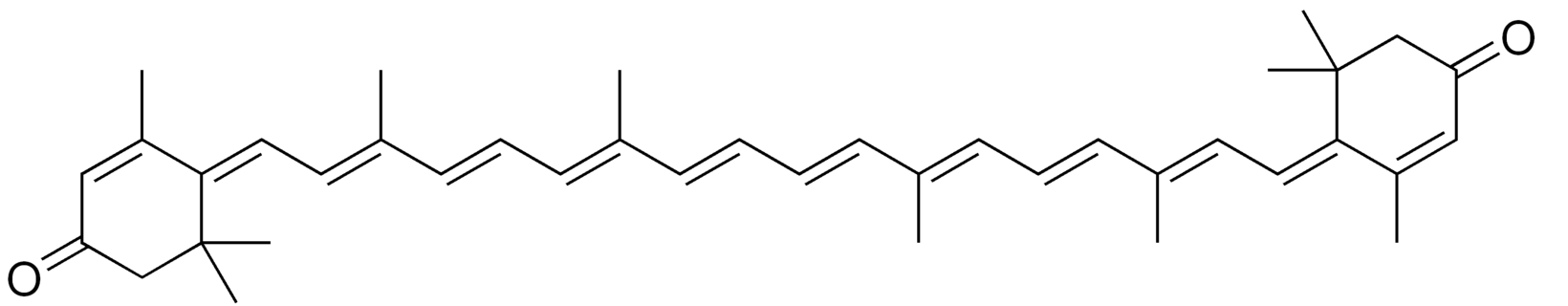 Rhodaxanthin
Rhodaxanthin
Nostoxanthin ((2,3,2′,3′)-β,β-carotene-2,3,2′,3′-tetrol) is a yellow carotenoid biosynthesized from zeaxanthin by the addition of two hydroxyl groups. It has been isolated from some prokaryotes including some species of Cyanobacteria: the bacteriochlorophyll-a containing bacterium Sandarakinorhabdus limnophila, photosynthetic bacteria, the non-marine Brevundimonas sp., and most Sphingomonas species. The strain Sphingomonas sp. SG73 was shown to produce highly pure nostoxanthin of approximately 97% (area%) of the total carotenoid production (Kikukawa H et al., Mar Drugs 2021, 19, 274).
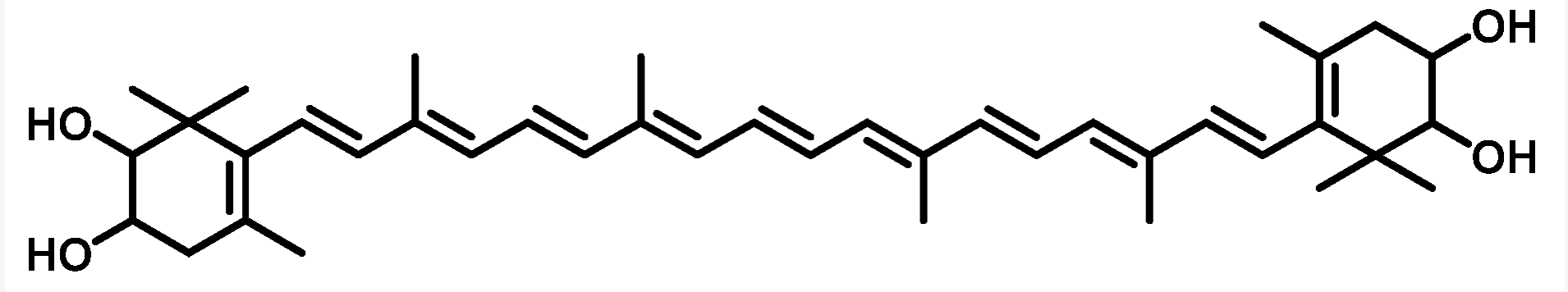
Nostaxanthin
Siphonaxanthin is a rare marine carotenoid abundant in green algae such as Codium fragile, Umbraulva japonica, and Caulerpa lentillifera (Sugawara T et al., Marine Drugs, 2014, 12, 3660). To date, antiangiogenesis, anti degranulation, and anti obesity effects of SPX have been reported (Li ZS et al., J Nutr 2015, 145, 490). This carotenoid exist also as an ester form (siphonein) on its C-19 hydroxyl group. It has been shown that siphonaxanthin Inhibits lipogenesis in hepatocytes via the suppression of liver X receptor α activity (Zheng J et al., Lipids 2018, 53, 41). It has been reported that siphonaxanthin elicited a potent inhibitory effect on hepatic de novo lipogenesis, and an anti-obesity effect in both 3T3L1 cells and KKAy mice. Thus, it was hypothesized that consumption of siphonaxanthin could improve metabolic disorders including hepatic steatosis and systemic adiposity, as well as ameliorate somatic stress under obese conditions (Zheng J et al., Nutr Res 2020, 77, 29). A review has focused on siphonaxanthin as a bioactive compound and outlines the evidence associated with functionality (Sugawara T et al., Mar Drugs, 2014, 12, 3660).
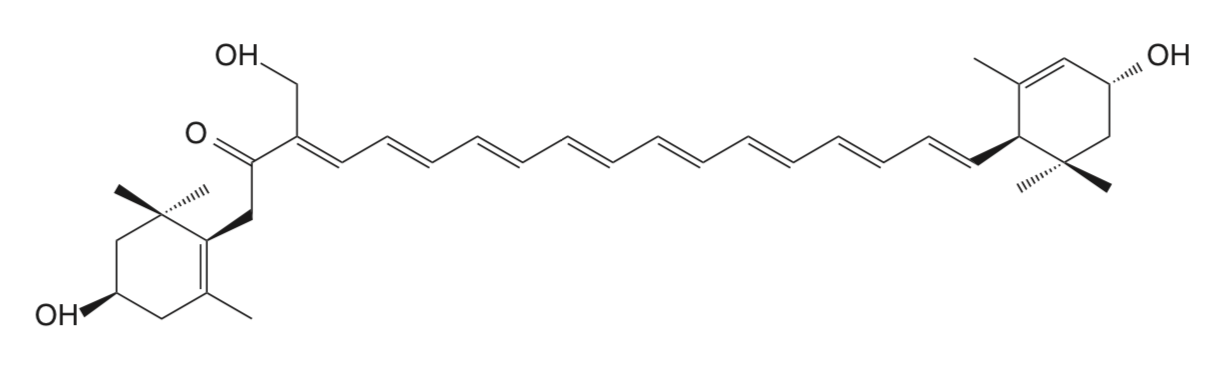
Siphonaxanthin
Loroxanthin is one of the xanthophylls of Chlamydomonas reinhardtii, which is formed by the hydroxylation on the carbon chain of the methyl group at C9 of lutein by an unknown enzyme. It is widespread among Chlorophyte, Euglenophyte, and Chlorarachniophyte (Takaichi S, Mar Drugs 2011, 9, 1101–1118).
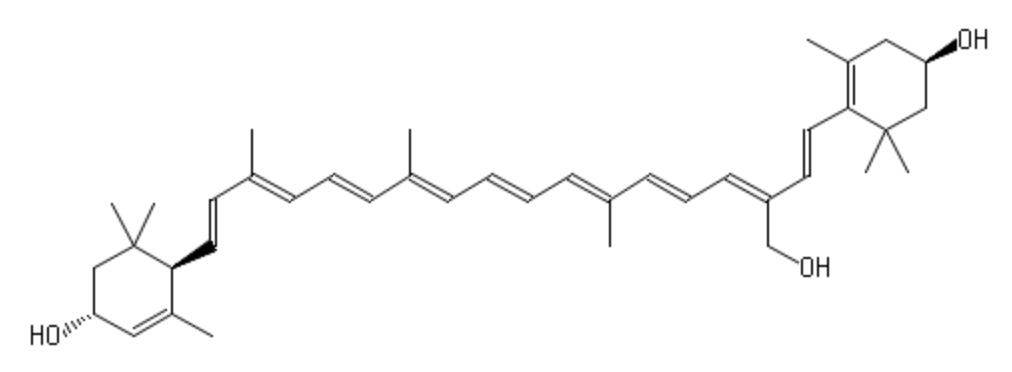
Loroxanthin
An important review has discussed the place of carotenoids in different market sectors, the methods for commercial production, the evidence supporting the health claims made by different industry sector for the most commercially valuable carotenoids on the market: β-carotene, lycopene, lutein, zeaxanthin and astaxanthin (Berman J. et al., Phytochem Rev 2015, 14, 727).
Ketocarotenoids belong to the xanthophyll group and are quite unique in nature. Among these compounds, echinenone and canthaxantin are abundant in cyanobacteria are characteristic of cyanobacteria (Takaichi S et al., Cell Mol Life Sci 2007, 64, 2607).
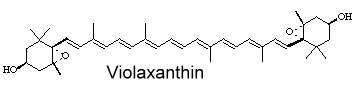
Violaxanthin is an epoxidized derivative of antheraxanthin (hydroxylated cryptoxanthin) and forms with zeaxanthin the xanthophyll cycle that is said to protect the photosynthetic system of plants against damage by excess light. The mature endocarp of the sweet orange fruit abundantly contained violaxanthin mono- and diesters (Lux PE et al., J Agric Food Chem 2019, 67, 13164).
Two cycloaddition products of trans-violaxanthin with a-tocopherol have been isolated from seeds of Pittosporum tobira and their structures elucidated (Fujiwara Y et al., Tetrahedron Lett 2001, 42, 2693). These C69 carotenoids were named pittosporumxanthins. Their global structure is given below.
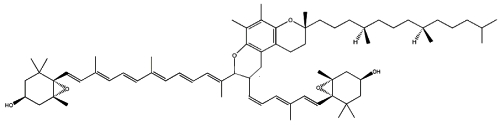
Pittosporumxanthin
Further investigations have revealed the existence of six other forms based on addition of α-tocopherol with antheraxanthin, neoxanthin or violaxanthin (Maoka T et al., J Nat Prod 2008, 71, 622).
Diatoxanthin is also an acetylenic carotenoid of the xanthophyll family. This pigment, described in 1978, is a specific marker for the Chromophyte algae and, in the marine phytoplankton, is present in diatoms, dinoflagellates and prymnesiophytes (Demers et al., Mar Ecol Prog 1991, Ser. 76: 119). The formation of diatoxanthin (the epoxy-free form) from its parent compound, diadinoxanthin, is induced by high light intensities. Some authors consider diatoxanthin as the only pigment acting as a photoprotectant (Paerl et al., Limnol Oceanogr 1983, 28, 847).
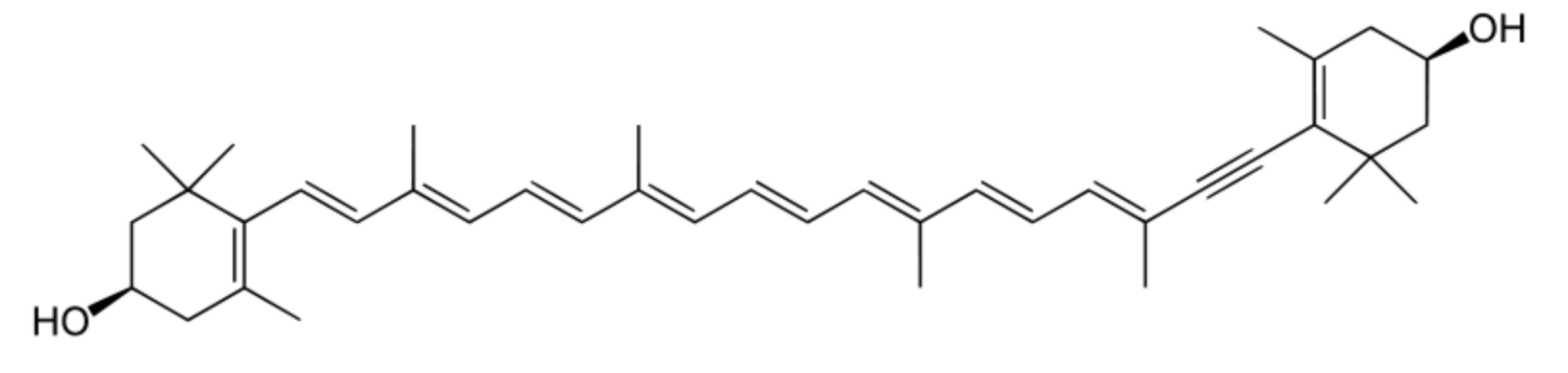 Diaoxanthin
Diaoxanthin
Investigations have proved that this compound displays interesting photochemopreventive activity in being able to lower intracellular oxidative stress and inflammation signaling induced by UV stress, focusing on possible developments for biomedical and cosmeceutical future applications (Pistelli L et al., Mar Drugs 2021, 19, 354).
Fucoxanthin is an allenic carotenoid with a 5,6-monoepoxide group. Although of limited occurrence in foods, fucoxanthin is among the most abundant carotenoids in nature. It is found in edible brown algae, largely consumed in Asian countries, and in diatoms. This compound contributes more than 10% of the estimated total production of carotenoids in nature. Fucoxanthin was first isolated by Willstätter R et al. (Ann 1914, 404, 237).
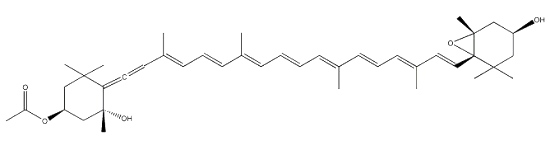
Fucoxanthin
This carotenoid has shown interesting biological properties, such as anticarcinogenetic effects, anti-inflammatory effects and radical scavenging activity. Consequently, products containing fucoxanthin are widely used in the market as functional food supplements, Furthermore, it has been shown that fucoxanthin has an anti-obesity effect in connection with the expression of the uncoupling protein UCP1 in white adipose tissue (Maeda H et al., Biochem Biophys Res Comm 2005, 332, 392; Miyashita K, Lipid Technol 2009, 21, 186). Its ability to enhance the docosahexaenoic acid concentration in the liver of treated obese mice remains to be examined more thoroughly (Tsukui T et al., J Agric Food Chem 2007, 55, 5025). Fucoxanthin was also shown to promote the conversion efficiency of alpha-linolenic acid in feeding to docosahexaenoic acid in quail egg yolk (Guo WL et al., Food Chem January 2025, 142915).
A great part of the biological properties of fucoxanthin may be related to its ability to take up peroxynitrite through the formation of nitrofucoxanthin and the inhibition of the nitration of tyrosine by peroxynitrite (Tsuboi M et al., J Agric Food Chem 2011, 59, 10572). Therefore, fucoxanthin may have the potential to reduce the risk of cancer induced by reactive nitrogen species. Among 15 carotenoids evaluated, fucoxanthin was reported to cause a remarkable reduction in the growth of prostate cancer cells (Hosokawa M et al., Food Sci Technol Res 1999, 5, 243–246, 1999).
Research have shown that fucoxanthin improves Aβ-induced-oxidative stress in microglia cells, suggesting that it might help Alzheimer disease treatment (Pangestuti R et al., J. Agric Food Chem 2013, 61, 3876). A review offered a comprehensive and holistic approach to the analysis of the biopharmaceutical and nutraceutical potential of fucoxanthin in the context of neurodegenerative diseases (Yordi EG et al., Marine Drugs 2024, 22(7), 327).
A review focused on the latest progress in research on fucoxanthin pharmacological activity and their underlying mechanisms (Xiao H et al., Marine Drugs 2020, 18, E634). Fucoxanthin was shown to be a potential therapeutic agent for osteoclast-related skeletal disorders including osteoporosis and rheumatoid arthritis (You-Jung Ha et al., Mar Drugs 2021, 19, 132).
Studies have examined the mechanism of inhibition of melanoma by fucoxanthin and its digestion products in cell models constructed using human malignant melanoma cells (A375) and keratinocytes (HaCaT) (Lu Y et al., Food Chemistry 25 August 2024, 141003).
A comprehensive review of sources and applications of fucoxanthin in biology and medicine may be consulted (Pajot A et al., Mar. Drugs 2022, 20, 222). Two updates of fucoxanthin pharmacological insight have been released in 2022 (Mohibbullah Md et al., Mar Drugs 2022, 20(5), 279; Tsz-Ying Lau et al., Mar Drugs 2022, 20(6), 370).
There is rising evidence that fucoxanthin plays a preventive and therapeutic role in neurological disorders. A review has focused on the metabolism, bioavailability, and blood–brain barrier penetration of fucoxanthin, on its neuroprotective potential in neurodegenerative diseases, cerebrovascular diseases, and psychiatric diseases as well as other neurological disorders (Chen Y et al., J Agric Food Chem 2023, 71 3599).
In 2017, a group reported the cancer-preventive effect of fucoxanthinol which is an intestinal metabolite of fucoxanthin. Notably, orally consumed fucoxanthin undergoes a deacetylation in the small intestine (under action of intestinal esterases), which results in fucoxanthinol. This has been observed in both mice and humans (Terasaki M et al. J Clin Biochem Nutr. 2017, 61, 25).
Neoxanthin is another common allenic carotenoid, widely distributed in higher plants and algae, which was first isolated from the green leaves of barley in 1938 by Strain HH.
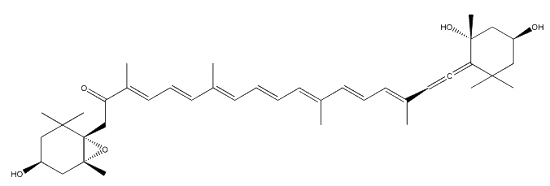
Neoxanthin
Neoxanthin is thought to be a part of the light harvesting complexes in thylakoids and as a precursor to the plant growth hormone abscisic acid. More than 40 allenic carotenoids have been described in vegetals and accumulated in animals (Dembitsky VM et al., Prog Lipid Res 2007, 46, 328). An unusual carotenoid ester was identified in fresh mango lipids as violaxanthin dibutyrate (Pott I et al., Phytochemistry 2003, 64, 825). Another unusual acetylenic carotenoid was discovered and identified in a marine sponge (Prianos osiros) (Rogers EW et al., J Nat Prod 2005, 68, 450).
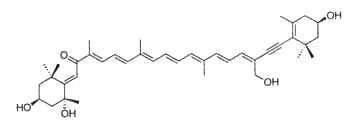
That new molecule was shown to be strongly cytotoxic toward cultured human colon tumor cells.
Salinispora tropica, a marine actinomycetes, is capable of producing orange carotenoids in the vegetative phase of growth. One of the orange carotenoids was identified as sioxanthin. It is a C40-carotenoid, glycosylated on one end of the molecule and containing an aryl moiety on the opposite end (Richter TKS et al., Environ Microbiol
2015, 17, 2158). It seems to have potent antioxidant properties (Jezkova Z et al., Marine Drugs 2021, 19/9).
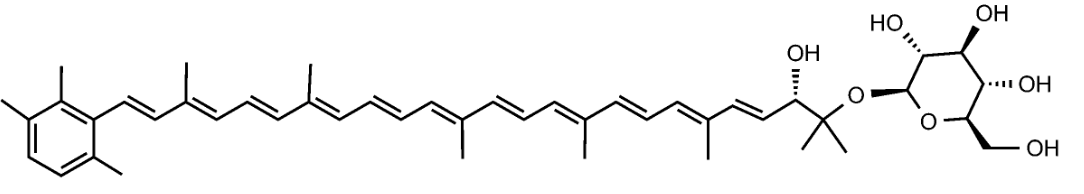 Sioxanthin
Sioxanthin
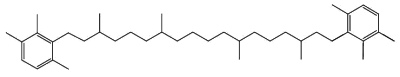
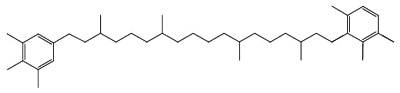
C50-carotenoids, including bacterioruberin which is present in halophilic Archaea, characterize the colored saltern ponds (Lutnaes BF et al., J Nat prod 2002, 65, 1340).
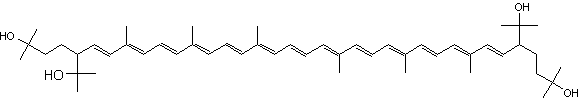
Bacterioruberin
Bacterioruberin possesses 13 carbon double bonds and 4 hydroxyl groups at the terminal ends that confer it a high antioxidant potential, even greater than the reference antioxidant β-carotene under certain conditions. Its function in the cell has been related to UV protection and membrane stability. Bacterioruberin-rich extracts from various species of haloarchaea, including the genera Haloarcula, Halobacterium, Halorubrum, and Haloferax, have displayed diverse bioactive properties in vitro, such as antibacterial, neuroprotective, anti-inflammatory, antiglycemic, and antilipidemic activities (Mapelli-Brahm P et al., Mar Drugs 2023, 21, 340). That carotenoids has shown considerable potential for health-promoting effects., but this field of research is still relatively underexplored.
Haloarchaeal C50 carotenoids have caught the attention of many researchers due to their particular structures, which would provide them with higher scavenger activity than their C40 counterparts (Giani M et al., Mar Drugs 2019, 17, 524). However, the actual beneficial effect of these natural antioxidants on human health is yet to be determined (review in Giani M et al., Mar Drugs 2021, 19, 594). A summarized knowledge on their potential use as anticancer molecules shedding new light on the design of drugs and new therapies based on natural compounds (Martínez-Espinosa RM, Mar Drugs 2024, 22(10), 448).
Although green leaves contain unesterified xanthophylls, in ripe fruit, most are esterified with fatty acids. However, those of a few fruits, particularly those that remain green when ripe, such as kiwi, undergo limited or no esterification. Carotenoid mono- and diesters were identified in mandarin essential oil (Giuffrida D et al. Flavour Fragr J 2006, 21, 319), in Haematococcus pluvialis (Breithaupt DE et al., J Agric Food Chem 2004, 52, 3870) and in the Antarctic krill Euphausia superba (Takaichi S et al. Comp Biochem Physiol B 2003, 136, 317).
Carotenoids fatty acid esters were also described in the carapace of the spiny lobster Panulirus japonicus (Maoka T et al., J Oleo Sci 2008, 57, 145). Astaxanthin, adonixanthin, and pectenolone were esterified with fatty acids with a large range of chain length and unsaturation.
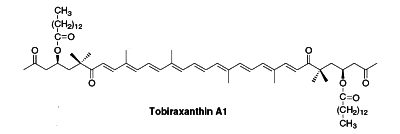
Several esterified seco-carotenoids, the tobiraxanthins, have been isolated from the seeds of Pittosporum tobira (Fujiwara Y et al., Tetrahedron Lett 2002, 43, 4385). Two fatty acid molecules (lauric or myristic acid) acylated the carotenoid part. One of these compounds is shown below (Tobiraxanthin A1).
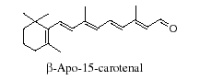
Apocarotenoids are carotenoid derivatives formed by the removal of fragments of the carbon backbone from either or both ends of a C40 precursor such as lycopene or β,β-carotene. These modification originate in the oxidative degradation at the level of the terminal rings. They may be the result of nonspecific mechanisms (lipoxygenase, photo-oxidation) as well as of specific mechanisms (dioxygenases). They have significant roles as developmental and environmental response signals. They also make important contributions to flavor and nutritional quality of foods (fruits, tea, wine) and tobacco.
Two well-known apocarotenoids are bixin and crocetin which have economic importance as pigments and aroma in foods.
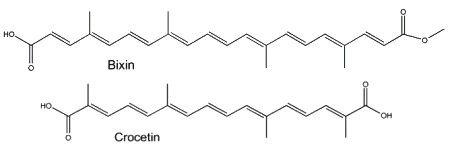
Bixin (cis- or trans-bixin) is one of several highly colored molecules extracted from annatto seed coats (Bixa orellana) and used as a food coloring and cosmetic compound since pre-Colombian times. It was determined that lycopene is the precursor of bixin (Bouvier F et al., Science 2003, 300, 2089). A review of the molecular characterization of bixin may be consulted (Ramamoorthy S et al., Ind Crops Prod 2010, 32, 48). Bixin demonstrated significant anti-oxidant, anti-inflammatory, and anti-lipidemic effects (Puri S et al., Food Biosci January 2025, 105893).
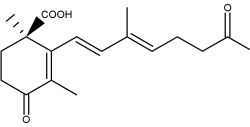
Crocetin, occurring as various glycosyl esters, is the coloring principle of saffron, pollen harvested from Crocus sativus and in fruits of Gardenia jasminoides. This apocarotenoid originates in the rupture of zeaxanthin (Bouvier F et al., Plant Cell 2003, 15, 47). This carotenoid may have several physiological properties in decreasing the effects of physical fatigue in healthy men and in improving the quality of sleep. The main coloring compound of saffron is crocin, one crocetin molecule esterified with two sugars (gentiobiose), the final product being water-soluble. α-Crocin may make up more than 10% of dry saffron’s mass.
Saffron as a natural product has long been used to impede and treat different disorders including cardiovascular disease (Ghaffari S et al., Biomed Pharmacother 2019, 109, 21). More recently, saffron was shown to be efficient in treating neuro-cognitive disorders (Cerdá-Bernad D et al., Nutrients 2022, 14, 5368) and could be a source of nutrients for health and for the treatment of neuropsychiatric and age-related diseases (El Midaoui A et al., Nutrients 2022, 14, 597). Clinical trials are needed to support the evidence of saffron and of its main bioactive components (e.g., crocin, crocetin) as a therapeutic product to combat neurological disorders. It has been reported that crocetin could be the true active component of saffron, functioning as a GPR40/120 agonist with potential implications in antidiabetic interventions (Zhao X et al., Nutrients, 2023, 15, 4774).
A derivative, sodium transcrocetinate, was shown to improve oxygenation in hypoxic tissues. It has been reported to exert a protective effect against cerebral and heart ischemia/reperfusion injury (Chang G et al., Int Immunopharmacol 2019, 71, 361).
As crocin is widely used in the food, cosmetics, and pharmaceutical industries, researchers have turned their attention to the heterologous production of crocin in a variety of hosts (Escherichia coli, Saccharomyces cerevisiae, microalgae, and plants) (Zhou J et al., Mar Drugs 2024, 22, 22).
Two other C26 and C30 apocarotenoids have been characterized in the seeds of Ditaxis heterantha (a native Euphorbiaceae of Mexico), heteranthin and ditaxin (Mendez-Robles MD et al., J Nat Prod 2006, 69, 1140). The presence of these products explains the use of the Ditaxis seeds as a natural food flavor and coloring.
Apocarotenoids
Carotenoids are in constant turnover involving continuous biosynthesis and catabolism, and are substrates for the carotene cleavage dioxygenases that catalyze the cleavage of carotenoids to apocarotenoids. These enzymes cleave a broad range of carotenoids, such as lycopene, ß-carotene, δ-carotene, zeaxanthin, violaxanthin, and neoxanthin, to generate aldehydes and ketones, the ionones, that are volatile aroma compounds (Auldridge M.E. et al., Curr Opin Plant Biol 9, 315), known also as rose ketones. Carotenoids that do not contain the β-ionone moiety cannot be converted to retinol, and thus have no vitamin A activity.
As an example, these metabolites originate from large carotenoid molecules found in grapes, such as ß-carotene and lutein. They accumulate during ripening, but break down into smaller compounds as the grapes reach maturity.
These secondary metabolites are of special interest because of their possible biological functions including herbivore feeding deterrence. It was shown that several volatile products are present after enzymatic degradation. Among them, the so-called norisoprenoids, the C13-cleavage products of carotenoids, have extremely low detection threshold values (some ng per liter of water for ionone and damascone).
Various bioassays demonstrated that ß-ionone has a strong repellent effect toward both the flea beetle and the spider mite, and significant oviposition deterrence to whiteflies. In contrast, dihydro-ß-ionone had attractant properties, especially to the crucifer flea beetle, while α-ionone did not show any significant activity (Cáceres L.A. et al., J Chem Ecol 42, 107). It may be expected that modified plants producing natural compounds with repellent or attractive effects will be exploited to reduce reliance on synthetic pesticides. Norisoprenoids are present in grapes in the form of glycosidic precursors. Their hydrolysis (enzymatic or chemical) leads to the formation of odorous compounds, aglycones, which once released contribute to the aroma of the wine. Among the most important norisoprenoids are β-damascenone, β-ionone, 1,1,6,-trimethyl-1,2-dihydronapthalene and vitispiran. β-Damascenone is generally present in all wines (!ncluding goji wines: Zheng Y et al., Food Chem 2024, 140803) above its detection threshold and plays the role of enhancer of the fruity aroma, β-ionone being responsible for the violet notes.
There is an enormous interest in apocarotenoids in detergent, food and perfume industry (Carotenoid-derived aroma compounds. Winterhalter P and Rouseff RL Eds, ACS Symp Series, v.802, 2002).
The ionones are a series of closely related chemical substances that are part of a group of compounds known as rose ketones, which also includes damascones and damascenones. They are aroma compounds found in a variety of essential oils, including rose oil. β-Ionone is a significant contributor to the aroma of roses. Carotenoids that do not contain the β-ionone moiety cannot be converted to retinol, and thus have no vitamin A activity. Numerous studies have highlighted the importance of β-ionone as β-ionone has protective effect against gastric, prostate, colon, and breast cancers. Research have demonstrated that it was also able to Inhibit skin cancer tumorigenesis (Yoon YE et al., Mol Nutr Food Res 2024, 68, 2400085).
α-Ionone
α-Damascone
A peroxidase from edible fungi was shown to be a key enzyme able to degrade carotenoids into important flavor compounds (ionone, cyclocitral, terpineol….) (Zorn H et al., Biol Chem 2003, 384, 1049). A review of the oxidative remodeling of plastid carotenoids initiated by specific dioxygenases has been released by Camara B et al (Arch Biochem Biophys 2004, 430, 16).
Furthermore, apocarotenoids act as visual or volatile signals to attract pollinating agents, and are also key players in plant defense. Studies have shown that the loss of cleavage enzymes induces the development of axillary branches, indicating that apocarotenoids convey signals that regulate plant architecture (Bouvier F et al., Tr Plant Sci 2005, 10, 187). It appears progressively that, even in man, apocarotenoids may exert biological activities. While the mechanism of action of these compounds is not fully known, they have profound effects on gene expression through nuclear receptors (Eroglu A et al., J Lipid Res 2013, 54, 1719).
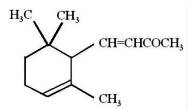
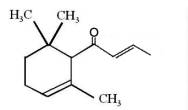
It is generally accepted that oxidation of carotenoids begins with epoxidation and cleavage to apocarotenals prior a transformation into other derivatives. Several epoxycarotenoids and apocarotenals were observed in experimental oxidation models but also some were identified in processed foods (Rodriguez EB et al., Food Chem 2007, 101, 563). The two compounds shown below were detected in fruit extracts :
Another apocarotenal, trans-β-apo-8′-carotenal, is found in spinach and citrus fruits. It has a low pro-vitamin A activity and is used in pharmaceuticals and cosmetic products. This apocarotenal is also used as an additive (E160e) legalized by the European commission for human food.
trans-β-Apo-8′-carotenal
The enzymatic cleavage of β-cryptoxanthin or zeaxanthin by carotenoid cleavage dioxygenase 4 gives rise in the flavedo of citrus fruit to β-citraurin (Ma G et al., Plant Physiol 2013, 163, 682). β-citraurin and its esters were found to be the unique flavedo (the outer colored layer of the mesocarp) constituents of mature fruit (Lux PE et al., J Agric Food Chem 2019, 67, 13164).
β-Citraurin
Apolycopenals have been reported in laboratory animals consuming high lycopene diets. Apo-8′- and apo-10′-lycopenal were observed in the hepatic tissue of rats consuming a high lycopene diet (Gajic M et al., J Nutr 2006, 136, 1552). These products are not limited to mammalian systems but were also identified in extracts of tomato. Thus, apo-6′-, apo-8′-, apo-10′-, apo-12′-, and apo-14′-lycopenals were detected and quantified in tomato (6.5 mg/100 g) (Kopec RE et al., J Agric Food Chem 2010, 58, 3290), apo-12′-carotenal being the most abundant. The presence of multiple apolycopenals in the plasma of humans consuming tomato juice has been documented. This evidence suggests that these products may in fact be absorbed from the food and not solely a product of metabolism in vivo.
Apo-12′-carotenal
Abscisic acid is an end product of neoxanthin or violaxanthine peroxidation and reduction giving an apocarotenal with a short side chain (5 carbons), followed by a final oxidation into an acid form (Seo M et al., Trends Plant Sci 2002, 7, 41).
Trisporoids, derived from b-carotene, regulate the recognition between mating partners, early sexual morphogenesis and development in zygomycete fungi. They are released from the hyphae (sexual cells), exerting their physiological effects upon compatible mating partners. Trisporic acid and some precursors directly influence the transcription of genes involved in sexual development.
Trisporic acid
The connection between b-carotene and trisporic acid was early established when trisporic acid was established as a substance produced by mated cultures of strains of Blakeslea trispora and Mucor mucedo (van den Ende H., Nature 1967, 215, 211) and as a metabolite of b-carotene after using 14C labeled molecules (Austin DJ et al., Experientia 1970, 26, 348). As for several other b-carotene derived signal compounds, the first step in trisporoid synthesis is the oxidative cleavage of b-carotene (b-carotene oxygenases).
Strigolactones are signaling molecules that play a double role in the rhizosphere as host detection signals for arbuscular mycorrhizal fungi and root parasitic plants (Bouwmeester HJ et al., Trends Plant Sci 2007, 12, 224). In addition to their important role as rhizosphere signaling compounds, it has recently been demonstrated that strigolactones also act as new hormones inhibiting shoot branching in plants and hence are involved in the regulation of above-ground plant architecture (Gomez-Roldan V et al., Nature 2008, 455, 189; Umehara M et al., Nature 2008, 455, 195; Tsuchiya Y et al., Curr Opin Plant Biol 2009, 12, 556). The dual function, as hormones and as rhizosphere signaling molecules, makes strigolactones unique among plant hormones. Exogenous strigolactones have been shown to alleviate drought stress in wheat (Triticum aestivum L.) by promoting cell wall biogenesis to optimize root architecture (Song M et al., Plant Physiol Biochem 2023, 204, 108121). Strigolactones appear thus as promising biomolecule for oxidative stress management (review in: Mansoor S et al., Plant Physiol Biochem 2024, 206, 108282).
Since the isolation of strigol as a germination stimulant for Striga lutea from roots of Gossypium hirsutum (Cook CE et al., J Am Chem Soc 1972, 94, 6198), more than ten strigolactones have been identified.
The 5-deoxystrigol was considered as the precursor of all other strigolactones (Orobanchol, strigol) but more recent works suggest that another derivative, bryosymbiol, identified in the bryophyte Marchantia paleacea but also in vascular plants, could be
the common ancestor of strigolactones for all land plants (Kodama K et al., Nature Comm 2022, 13, 3974).
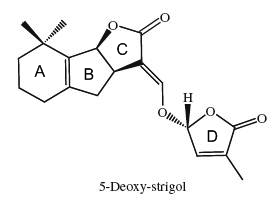
The structural core of the molecules consists of a tricyclic lactone (ABC part) that connects via an enol ether bridge to a butyrolactone group (the D-ring). All other strigolactones possess one or two methyl substituents on the A-ring and various combinations of hydroxyl or acetate substituents around the A- and B-rings. Seven novel strigolactones, named zealactones, have been isolated from maize (Charnikhova TV et al., Phytochemistry 2017, 137, 123).
It has been demonstrated that these terpenoids are derived from the carotenoid pathway (Matusova R et al., Plant Physiol 2005, 139, 920).
The biology of strigolactones has been reviewed (Ruyter-Spira C et al., Trends Plant Sci 2013, 18, 72 and Khosla A et al., Curr Op Plant Biol 2016, 33, 57). Their role in the plant defence system has been reviewed (Marzec M, Tr plant Sci 2016, 21, 900). A review offers useful structural insights into strigolactone perception, the precise molecular adaptations that define receptor-ligand specificities, and the mechanisms of their hydrolysis (Guercio AM et al., Phytochem Rev 2023, 22, 339).
Carotenoids may be linked to a sugar by a glycosidic link or an ester link. Such compounds are known for plant and bacteria. As an example, a glucoside of rhodopsin, found in the photosynthetic part of Rhodopseudomonas acidophila, is shown below.
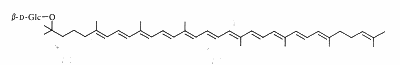
Salinixanthin is a glycosylated and acylated carotenoid associated with the protein xanthorodopsine which was isolated from the extremely halophilic eubacterium Salinibacter ruber living in salt pond in Spain (Lutnaes BF et al., J Nat Prod 2002, 65, 1340). This carotenoid has a keto group in the ring, a glycoside group and an acyl tail, probably immersed in the membrane (Balashov SP et al., Cell Mol Life Sci 2007, 64, 2323).
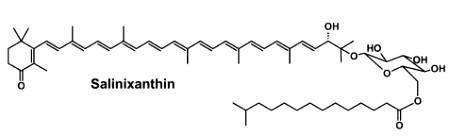
Staphyloxanthin is a carotenoid pigment that is produced by some strains of Staphylococcus aureus. Staphyloxanthin is a glucosyl ester of 4,4′-diaponeurosporen-4-oic acid, the glucose being also esterified with 12-methyl tetradecanoic acid (anteisopentadecanoic acid). This carotenoid is responsible for the characteristic golden color that gives its species name aureus). Staphyloxanthin also acts as a virulence factor. It has been shown that drugs inhibiting its production may weaken it and renew its susceptibility to antibiotics (Liu GY et al., J Exp Med 2005, 202, 209).
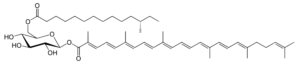
Staphyloxanthin
Astaxanthin diglucoside diesters have been determined in lipid extracts of the snow alga (Chlamydomonas nivalis) (Rezanka T et al., Phytochemistry 2008, 69, 479). The C-3 hydroxyl group of astaxanthin is glucosylated and the C-6 hydroxyl group of the glucosyl moiety is esterified with specific fatty acids. Among these fatty acids (14 species were detected), the most abundant were 16:3, 16:4, 18:1, 18:4 and 18:5.
Carotenoids are said to have antioxidant properties, as tocopherols, and thus may prevent the oxidation of the lipid moieties of LDL (low density lipoproteins) which renders these lipoproteins atherogenic.
To know more about their antioxidant properties, consult the VERIS site
The web site of the International Carotenoid Society may be consulted for further information on carotenoids.
A very informative guide to carotenoid analysis in foods has been released by Rodriguez-Amaya DB.
Devenez membre et participez au développement de la Lipidomique au XXIème siècle.
S'inscrire