
Cyclohexyl fatty acids were first found in butter (Shogt JC et al., 1965, 6, 466) and then in sheep fat (Hansen RP et al., J Sci Food Agric 1967, 18, 225) and rumen bacteria (Hansen RP, Chem Ind 1967, 39). The most common cyclohexyl acids are 11-cyclohexyl-C11 and 13-cyclohexyl-C13 (see below).
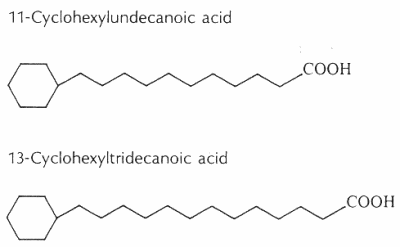
The content of w-cyclohexyltridecanoic acid varied from 0.0% to 0.15% of milk fat, and it was higher in milk samples from cows fed with diets richer in cereal meals (Marseglia A et al., Food Chem 2013, 140, 711).
While these w-cyclohexyl acids were detected in a mesophile bacteria Curtobacterium pusillum (Kawagushi A et al., J Biochem 1986, 99, 1735), they are curiously more abundant in thermophile bacteria. Thus, they are, in equal amounts, the major components (about 65%) of the fatty acids in Bacillus acidocaldarius (= Alicyclobacillus), a gram-positive rod-shaped prokaryote found in acid hot springs up to 65°C (De Rosa M et al., Chem Commm 1971, 1334). They were also found in ten strains of acido-thermophilic bacteria isolated from different Japanese hot springs (Oshima M et al., J Biol Chem 1975, 250, 6963). These fatty acids were found in the esterified form in glyceride type lipids, amounted to 74 to 93% of the total fatty acids in the bacteria, and were shown to be formed from glucose and shikimic acid. More recently, cyclohexyl undecanoic acid was shown to be the major cellular fatty acid in Propionibacterium cyclohexanicum, a new acid-tolerant bacteria isolated from spoiled orange juice (Kusano K et al., Int J Syst Bacteriol 1997, 47, 825).
Some C17 and C19 cyclohexyl alkanoic acids have been identified in acidothermophilic bacteria in hot springs. Furthermore, a series of alkyl cyclohexanes and cyclohexyl alkanoic acids were detected in lacustrine saline petroleum samples from Brazil (Nascimento LR et al., Org Geochem 1999, 30, 1175). The origin of these compounds is still unknown, but a correlation was made with the presence of Alicyclobacillus in the formation water and oil samples (Rodrigues DC et al., Org Geochem 2005, 36, 1443).
During heating of vegetal oils, linolenic acid gives rise to dienoic acids containing a cyclohexenyl or a cyclopentenyl ring. Similarly, deodorization of fish oil rich in eicosapentaenoic and hexaenoic acids generates about 15 cyclohexyl and cyclopentyl fatty acids with a 20 and 22 carbon chain, respectively (Berdeaux O et al., J Chromatogr A 2007, 1138, 216). One of the most abundant cyclic monomers formed from EPA (8-(2′-hexylcyclohexyl) octanoic acid) is shown below.
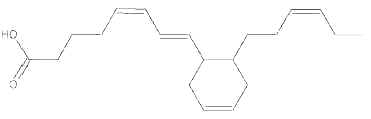
8-(2′-hexylcyclohexyl) octanoic acid
A more complex fatty acid containing a highly functionalized cyclohexanone has been isolated from the endophytic fungi Pestalotiopsis spp and Monochaetia sp living in the thalli of lichens (Li JY et al., Phytochemistry 2001, 56, 463). The compound, named ambuic acid, contains a hexa-substituted cyclohexanone moiety, as it was found in a tetracycline antibiotic.
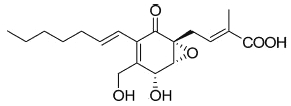
Ambuic acid
Ambuic acid has displayed antifungic activity against human pathogenic fungi. Several secondary metabolites of ambuic acid have been described in the same fungus (Pestalotiopsis sp) (Ding G et al., J Nat Prod 2009, 72, 182). Ambuic acid and one of its metabolites displayed anti-microbial activity against the Gram-positive bacterium Staphylococcus aureus.
zaragosic acids are a complex of fatty acids from fungi which possess an unique tricarboxylated 4,8-dioxabicyclo[3.2.1]octane core. They are potent inhibitors of S. cervisiae, fungal and mammalian squalene synthase and therefore inhibitors of sterol synthesis (Bergstrom JD, et al., Proc Natl Acad Sci U S A 1993, 90, 80).

Zaragosic acid A
Devenez membre et participez au développement de la Lipidomique au XXIème siècle.
S'inscrire