
Carboxylic acids occur in many molecular forms. At first It must be recalled that if the majority of the fatty acids found in lipids are monocarboxylic acids, some of them are dicarboxylic and constitute important metabolic or oxidation products of the previous ones.
Several hundreds of forms have been identified but the number occurring frequently in the common lipids is much fewer (from 10 in plants to about 20 in animal tissues). Several fatty acids, free or esterified (methyl butyrate, ethyl octanoate, dodecanoic acid…), belong to aroma compounds which are found in environmental or food systems (see the website: Flavornet). The analyzed fatty acid profiling of plants or microalgae have been used to classify them into distinct taxonomic orders with respect to their phylogenetic classification (Sahu A et al., Phytochemistry 2013, 89, 53). That experimental approach has been reported as a tool for studying the chemotaxonomic features in various species macro- and microalgae.
Fatty acid methyl and ethyl esters are known to be present in the plasma of patient with liver dysfunction following ethanol ingestion (Aleryani SL et al., Clin Chim Acta 2005, 359, 141; Politi L et al., Anal Biochem 2007, 368, 1).
Triglycerides from various vegetable oils give through transesterification a mix of fatty acid esters which is now used increasingly as a substitute of diesel fuel and is named Biodiesel.
The world production of fatty acids from the hydrolysis of natural fats and oils totaled about 4 million metric tons per year. Fatty acids are ultimately consumed in a wide variety of end-use industries (rubber, plastics, detergents…). As it is a good indication of the overall economic performance of a region, the consumption of fatty acids has tended to approximate the growth in the GNP of the region of their consumption. Fatty acids make up the greatest proportion of the current consumption of raw material in the chemical industry. The extent of the chemical reactions which are used to transform these renewable materials has been summarized (Bierman U et al., Angew Chem Int Ed 2000, 39, 2206). A short survey of oil crop platforms to be considered for either multi-purpose or technical oils production has been reviewed in 2009 (Carlsson AS, Biochimie 2009, 91, 665).
![]()
To describe precisely the structure of a fatty acid molecule, one must give the length of the carbon chain (number of carbon), the number of double bonds and also the exact position of these double bonds, this will define the biological reactivity of the fatty acid molecule and even of the lipid containing the fatty acids studied.
Most fatty acids are straight-chain compounds with the most frequently an even number of carbon atoms. Odd-numbered fatty acids are mostly frequent in bacteria and lower plants or animals (review in : Rezanka T et al., Prog Lipid Res 2009, 48, 206). Chain-lengths range is from 2 to 80 but commonly from 12 up to 24. With a chain length from 2 to 6 (or 4) they are called short-chain, from 8 (or 6) to 10 they are called medium-chain and 12 up to 24 they are called long-chain fatty acids. Their physical and biological properties are related to this partition in 3 classes. Very long chain fatty acids are also considered. They can be defined as fatty acids with 23 or more carbon atoms (Review in: Kyselová L et al., Prog Lipid Res 2022, 87, 101180).
An extensive review on the biochemical mechanisms of fatty acid elongation may be consulted for further information (Leonard AE et al., Prog Lipid Res 2004, 43, 36).
Fatty acids are simple in structure and even with their derivatives can be subdivided into well-defined families:
Among straight-chain fatty acids, the simplest are referred to as saturated fatty acids. They have no unsaturated linkages and cannot be altered by hydrogenation or halogenation. When double bonds are present, fatty acids are said unsaturated, monounsaturated (MUFA) if only one double bond is present and polyenoic (or polyunsaturated fatty acids = PUFA) if they have two or more double bonds generally separated by a single methylene group (methylene-interrupted unsaturation). In recent physiological works, the last class is used only for fatty acids with three up to six double bonds as those found in fish oil or in brain tissue. Some uncommon polyunsaturated fatty acids have two adjacent double bonds separated by more than one methylene group, they are named polymethylene-interrupted fatty acids.
In some animals, but mainly in plants and bacteria, fatty acids may be more complex since they can have an odd number of carbon atoms, branched chains or contain a variety of other functional groups, including acetylenic bonds, epoxy-, hydroxy– or keto groups and even ring structures (cyclopropane, cyclopropene, cyclopentene, furan, and cyclohexyl) or a coenzyme A moiety (acyl CoA).
Except fatty acyl-CoA, we have based our classification of fatty acids first on the type of carbon chain : either straight (or normal), or branched, or containing a carbon ring. In each category, subdivisions are created according to the functional groups substituted on the carbon chain.
![]()
To describe the unsaturated fatty acids, two ways are offered:
The chemist’s terminology:
The carbon atoms are counted from the carboxyl group which put the emphasis on the double bond closest to this group.
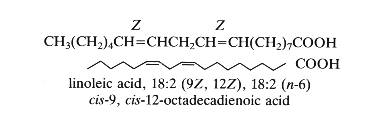
As an example: 18:2 D9,12 or cis-9, cis-12-octadecadienoic acid, the trivial name: linoleic acid. The double bonds have usually a Z (cis) configuration but can have also a E (trans) configuration.
The biochemist’s and physiologist’s terminology:
Holman RT proposed in 1964 a new numbering system for the unsaturation of fatty acids, the “omega nomenclature”. The double bonds are counted from the methyl group determining the metabolic family, noted by wx (w for the terminal carbon) or better n-x (n for the total number of carbon, x being the position of the distal double bond) . The other double bonds are deduced from the first one by adding 3 (this is the most frequent structure, non-conjugated fatty acids, but sometimes by adding 2, these double bonds are said conjugated).
Thus, linoleic acid or cis-9, cis-12-octadecadienoic acid is also named in the shorthand nomenclature 18:2 (n-6). This compound has 18 carbon atoms, 2 double bonds and 6 carbon atoms from the last double bond to the terminal methyl group. In the old literature it was designated 18:2w6.
18-6=12, 12-3=9, hence D9,12.
The International Commission on Biochemical Nomenclature agreed to the first form of this nomenclature because of its interest in describing the fatty acid metabolism.
A list of common (non-systematic) names for fatty acids (by Adlof R.O. and Gunstone F.D.) with their structure and source may be consulted in a page from AOCS (Common_Names_FattyAcids). The common names and the structures of many fatty acids may be found on the Lipidomics Gateway.
An important database available on Internet is the Lipid Bank for Web, a lipid database information retrieval system. It contains a lot of information about fatty acids and other lipid compounds. Additionally to physical and chemical data the database comprises information about the fatty acid composition of different oils.
Hundreds of fatty acids synthesized by thousands of plants and their phylogenetic relationships are documented in an internet data base, PlantFAdb (https://plantfadb.org/), which can be used to search fatty acid molecules or plants producing these fatty acids.
The most documented fatty acids data collection is that of the Institute for Chemistry and Physics of Lipids in Münster growing since 1970. This electronically searchable Database SOFA (Seed Oil Fatty Acids, http://sofa.mri.bund.de/) offers a variety of search routines to browse into about 110,000 individual data relating to more than 7,000 different plant species (Aitzetmüller K et al., Eur J Lipid Sci Technol 2003, 105, 92). About 500 different fatty acids are listed. The database allows to search for plant species, genera and families, for individual fatty acids (start by adding an asterisk after each entry) and combinations of fatty acids in their seed oils, and for their percentage contents. It contains literature references and numerous unpublished data. Moreover, fatty acid partial structures or functional groups can also be searched for, using the “delta-notation” system of chemists as described above. The use of the database is mostly straightforward and self-explanatory but several examples for search operations have been published to help anybody interested in seed oils and their fatty acid composition (Aitzetmüller K et al., Eur J Lipid Sci Technol 2003, 105, 92).
A comprehensive database of melting points of fatty acids, collected from the literature and from original measurements has been reported (Guendouzi A et al., Chem Phys Lipids 2012, 165, 1-6). A newly developed methodology for the fast selection of descriptors in quantitative structure–property relationships analysis of 62 fatty acids was also proposed.
A graphical chart of the oxidative degradation of fatty acids may be found on the Slideshare web site or the Medical Biochemistry Page.
![]()
Fatty acids can be subdivided into well-defined families
according to their chain structure and then to their other functional group :
A – Normal fatty acids (straight chain)
AA – Carbon chain without substituent
1 – Saturated fatty acids
2 – Monoenoic fatty acids
3 – Polyenoic fatty acids
Methylene-interrupted
Polymethylene-interrupted
Conjugated
Allenic acids
Cumulenic acids
AB – Carbon chain with substituent (other than methyl)
6 – Sulfur containing fatty acids
8 – Methoxy and acetoxy fatty acids
9 – Keto fatty acids
11 – Halogenated fatty acids (F, Cl, Br)
12 – Nitrated fatty acids
13 – Arsenic containing fatty acids
B – Branched-chain fatty acids
1 – Mono or multibranched chain fatty acids
2 – Branched methoxy fatty acids
3 – Branched hydroxy fatty acids (Mycolic acids)
C – Ring containing fatty acids
2 – Cyclobutane acids (ladderanes)
4 – Furanoid acids
5 – Cyclohexyl and hexenyl acids
6 – Phenyl and benzoic alkanoic acids
7 – Epoxy acids
9 – Lipoic acid
D – Fatty acyl-CoA
Devenez membre et participez au développement de la Lipidomique au XXIème siècle.
S'inscrire