
SULFUR CONTAINING FATTY ACIDS
– Thia fatty acids : sulfur-substituted fatty acid analogues are actively synthesized as they are reported to have important pharmacological properties (antiatherosclerosis and antioxidant).
They can have a variable number of carbon atoms and the sulfur atom in different position (3-thia or 4-thia).
The most commonly 3-thia fatty acids studied are presently:
dodeca thia acetic acid CH3-(CH2)11-S-CH2-COOH
tetradeca thia acetic acid CH3-(CH2)13-S-CH2-COOH
These fatty acid derivatives are reported to have triglyceride and cholesterol lowering effects in animal models (Skrede S et al., Biochim Biophys Acta 1997, 1344, 115).
The last one, tetradecylthioacetic acid (TTA) is used as a nutritional supplement. It has been demonstrated that it acts as a peroxisome proliferator-activated receptor alpha (PPARα) agonist and it increases mitochondrial fatty acid oxidation in vitro (Løvås, K et al., Diabetes, Obesity & Metabolism 2009, 11, 304) and modifies transport of fatty acids and HDL cholesterol in the small intestine (Lundåsen T et al., PLoS One 2020, 15:e0229322). TTA has been reported to have several physiological activities in rodents but
human clinical studies lead to divergent observations.
Isomeric epithio stearic acids have been described as minor constituents in canola oil. They were tentatively identified were the 9,12; 8,11; and 7,10 epithio stearic acids (Wijesundera RC et al., JAOCS 1988, 65, 959). The general formula is given below.
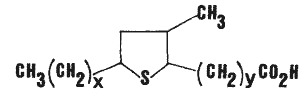
x = 5, 6 or 7 and y = 7, 6 or 5, respectively
– Sulfated fatty acids : New fatty acid derivatives have been isolated from the regurgitant of the grasshopper species Schistocerca americana. These compounds (named caeliferins) are composed of saturated and monounsaturated sulfated a-hydroxy fatty acids in which the w-carbon is substituted with a sulfated hydroxyl group (Alborn HT et al., PNAS 2007, 104, 12976). These compounds have a carbon chain of 15–20 carbons and are distributes in varying proportions. Of these, the 16:1 analog is predominant and is also the most active in inducing release of volatile terpenoid compounds when applied to damaged leaves of corn seedlings.
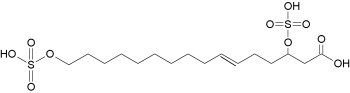
Caeliferin A 16:1
Devenez membre et participez au développement de la Lipidomique au XXIème siècle.
S'inscrire