
FATTY ALDEHYDES
Long-chain aldehydes are found in free form, but also in the form of vinyl ether (known as alk-1-enyl ether) integated in glycerides and phospholipids (plasmalogens).
The free aldehydes can be as fatty acids saturated or unsaturated. They have a general formula CH3(CH2)nCHO with n=6 to 20 or greater. The most common is palmitaldehyde (hexadecanal) with a 16 carbon chain.
trans-2-Dodecenal (eryngial) was identified as the main anthelmintic compound in Eryngium foetidum (Apiaceae) (Forbes WS et al., Parasitology 2014, 141, 269). This fatty aldehyde has also been shown to be responsible of the antiepileptic and other therapeutic activities of cilantro (Coriandrum sativum), another Apiaceae (Manville RW et al., FASEB J 2019, 33, 11349). It was demonstrated that t2-dodecenal is a highly potent KCNQ channel (neuronal voltage-gated potassium channel subfamily Q) activator. Curiously, Eryngium foetidum (culantro or Mexican coriander) and cilantro are used extensively and in ancient times in folk medicine as an anticonvulsant or antiepilectic. These data suggest a rich target profile for plant extracts in treating a variety of neuronal hyperexcitability disorders, such as the epilepsies, acute pain, neuropathic pain, migraine pain and some neurodegenerative and psychiatric disorders.
It must be noticed that an aldehyde function may be found at a terminal (ω) position while an acid function is present at the other end of the carbon chain (oxo fatty acids). These compounds have important signaling properties in plants.
Long-chain aldehydes have been described in the waxes which impregnate the matrix covering all organs of plants (Vermeer CP et al., Phytochemistry 2003, 62, 433). These compounds forming about 7% of the leaf cuticular waxes of Ricinus communis were identified as homologous unbranched aldehydes ranging from C22 to C28 with a hydroxyl group at the carbon 3. Long-chain 5-hydroxyaldehydes with chain lengths from C24 to C36, the C28 chain being the most abundant, were identified in the cuticular wax of Taxus baccata needles (Wen M et al., Phytochemistry 2007, 68, 2563). Long-chain aliphatic aldehydes with chain-length from C22 to C30 are also present in virgin olive oils, hexacosanal (C26) being the most abundant aldehyde (Perez-Camino MC et al., Food Chem 2012, 132, 1451).
Polyunsaturated aldehydes are among the most studied allelopathic compounds as they are commonly released into the aquatic environment by different phytoplankton species. Diatom-released polyunsaturated aldehydes were observed to affect phytoplankton community dynamics and structure, and showed inhibitory effects on the reproduction and development of marine invertebrates having allelopathic and anti-herbivory properties. Since their discovery, research has focused on their production by pelagic marine diatoms, and their effects on copepod egg production, hatching success, and juvenile survival and development. Eight species of benthic diatoms were studied for the production of the bioactive 2,4-heptadienal, 2,4-octadienal, and 2,4-decadienal (Johnson J et al., J Chem Ecol 2024, 50, 290).
The production of aldehydes by several Mediterranean macroalgae has been investigated providing the first evidence of potential allelochemical activity (Pezzolesi L et al., Phytochemistry 2021, 189, 112826). Results highlighted the potential production by macroalgae of a variety of aldehydes. Some species (i.e. Dictyopteris polypodioides and Ulva cf. rigida) were found to produce higher PUAs amounts than other.
Aldehydes may be produced during decomposition of fatty acid hydroperoxides following a peroxidation attack. Several aldehydes (hexanal, heptanal..) belong to aroma compounds which are found in environmental or food systems (see the website: Flavornet). Aldehydes (mono- or di-unsaturated) with 5 to 9 carbon atoms are produced by mosses (Bryophyta) after mechanical wounding (Croisier E et al., Phytochemistry 2010, 71, 574). It was shown that they were produced by oxidative fragmentation of polyunsaturated fatty acids (C18, C20). Trans-2-nonenal is an unsaturated aldehyde with an unpleasant odor generated during the peroxidation of polyunsaturated fatty acids. It participates to body odor and is found mainly covalently bound to protein in vivo (Ishino K et al., J Biol Chem 2010, 285, 15302).
Hexanal is the major wheat grass volatile which has also been identified as a component of the aggregation pheromone blend in a locust species, Schistocerca gregaria (Torto B et al., J Chem Ecol 1996, 22, 2273).
Two green leaf volatiles, (Z)-3-hexenal and (E)-2-hexenal, were identified in injured Arabidopsis and were shown to increase cytosolic Ca2+ concentration (Aratani Y et al., Nat Comm 2023, 14, 6236). These aldehydes trigger the expression of biotic and abiotic stress-responsive genes in a Ca2+-dependent manner. Studies revealed that calcium concentration increased instantly in guard cells and subsequently in mesophyll cells. These results suggest that these lipids in the atmosphere are rapidly taken up by the inner tissues via stomata, leading to cytosolic calcium increases and subsequent defense responses in Arabidopsis leaves.
It has been demonstrated that two endogenous aldehydes (hexanal and heptanal) may be used as lung cancer biomarkers in saliva samples (Azorin C et al., Anal chim Acta 2023, 1271, 341435). These results reveal the prospect of the method used (gas chromatography coupled to mass spectrometry) as potential diagnostic tool for lung cancer by saliva analysis.
The recognition of odorous signals was demonstrated to be accomplished by several subsystems (olfactory receptors) of the global olfactory system. These results led to the hypothesis that in mammals the OR37 receptor subsystem may specifically recognise chemical compounds. In the search for suitable ligands it was found that these receptors are activated by long-chain aliphatic aldehydes (Bautze V et al., Chem Senses 2012, 37, 479). It was found that each of the OR37 receptor subtypes selectively responds to a different but very similar aldehyde, which differs only in chain length. Pentadecanal was found to be the best ligand for OR37A, hexadecanal for OR37B and heptadecanal for OR37C. A more recent study has demonstrated that hexadecanal appears to foster aggressive behavior in women and blunt it in men. Thus, that molecule expressed by the human body can influence human behavior, specifically the aggressive behavior (Mishor E et al., Sci Adv 2021, 5/47). Thus, women exposed to the aldehyde behaved 19% more aggressively in a special test, whereas men were 18.5% less aggressive.
It has been demonstrated that sea-lettuce (Ulva species) produces elevated amounts of volatile C10-polyunsaturated aldehydes (2,4,7-decatrienal and 2,4-decadienal) upon tissue damage. These aldehydes are derived from n-3 and n-6 polyunsaturated fatty acids with 20 or 18 carbon atoms including C20:5 n-3 (EPA), C20:4 n-6, C18:4 n-3, and C18:3 n-6. Evidences were presented that lipoxygenase-mediated (11-LOX and 9-LOX) eicosanoid and octadecanoid pathways catalyze the transformation of these fatty acids into short chain hydroxylated fatty acids (Alsufyani T et al., Chem Phys Lipids 2014, 183, 100).
Fatty aldehydes may be determined easily by TLC or gas liquid chromatography (follow that link). The most common method for the determination of aldehydes involves derivatization with an acidic solution of 2,4-dinitrophenylhydrazine to form corresponding hydrazones followed by HPLC separation and UV–VIS detection. An optimized derivatization procedure for the determination of aliphatic C1-C10 aldehydes has been described (Stafiej A et al., J Biochem Biophys Meth 2006, 69, 15).
Other short-chain aldehydes (octadienal, octatrienal, heptadienal) are produced via a lipoxygenase-mediated pathway from polyunsaturated fatty acids (mainly C16 and C20) esterifying glycolipids in marine diatoms (D’Ippolito G et al., Biochim Biophys Acta 2004, 1686, 100). In nature, processes producing the disruption of phytoplankton cells are viral infection, grazing or/and cell lysis during senescence. Heighteen species of diatoms have been shown to release unsaturated aldehydes (C7:2, C8:2, C8:3, C10:2, and C10:3) upon cell disruption (Wichard T et al., J Chem Ecol 2005, 31, 949). The analyzis of the spatial distribution of the aldehydes produced by the phytoplankton in the Atlantic Ocean surface has shown that the total potential fatty adehyde concentrations ranged from zero to 4.18 pmol from cells in 1 L with, besides octadienal and decadienal, heptadienal being the most common (Bartual A et al., Mar Drugs 2014, 12, 682).
Diatoms are responsible for the fixation of ca. 20% of the global CO2 and live associated with bacteria that utilize the organic substances produced by them. Research have shown that diatoms and bacteria interact mediated through the production and exchange of infochemicals. Among them, the most important are polyunsaturated aldehydes released by diatoms. It has been observed that the bacterial presence significantly enhanced diatom (Cyclotella cryptica) growth and the modulation of the percentage of released aldehydes in response to the presence of bacteria. Thus, diatoms could use released unsaturated aldehydes as a specific organic matter able to attract beneficial bacteria for constructing their own phycosphere, for more beneficial growth (Hernanz-Torrijos M et al., Mar Drugs 2023, 21, 571).
Several short-chain aldehydes were shown to induce deleterious effects on zooplankton crustaceans and thus limiting the water secondary production (birth-control aldehydes) (D’Ippolito G et al., Tetrahedron Lett 2002, 43, 6133). In laboratory experiments, three decatrienal isomers produced by various diatoms were shown to arrest embryonic development in copepod and sea urchins and have antiproliferative and apoptotic effects on carcinoma cells (Miralto A et al., Nature 1999, 402, 173). Later, the copepod recruitment in blooms of planktonic diatom was shown to be suppressed by ingestion of dinoflagellate aldehydes (Nature 2004, 429, 403). It was demonstrated that diatoms can accurately sense a potent 2E,4E/Z-decadienal and employ it as a signaling molecule to control diatom population sizes (Vardi A et al., PLoS Biol 2006, 4, e60). This aldehyde triggered a dose-dependent calcium transient that has derived from intracellular store. Subsequently, calcium increase led to nitric oxide (NO) generation by a calcium-dependent NO synthase-like activity, resulting in cell death in diatoms.
Myeloperoxidase-derived chlorinated aldehydes with plasmalogens has been reported. Thus, the vinyl-ether bond of plasmalogens is susceptible to attack by HOCl to yield a lysophospholipid and an a-chloro-fatty aldehyde (Albert CJ et al., J Biol Chem 2001, 276, 23733). For example, 2-chloro-hexadecanal is formed by HOCl attack on the plasmalogen 1-O-hexadec-1′- enyl-sn-glycero-3-phosphocholine. Similarly, 2-chloro-octadecanal is formed from 1-O-octadec-1′-enyl-sn-glycero-3-phosphocholine.

2-Chloro-hexadecanal
Both these chloro-fatty aldehydes have been detected in neutrophils activated with PMA (Thukkani AK et al., J Biol Chem 2002, 277, 3842) and in human atherosclerotic lesions (Thukkani AK et al., Circulation 2003, 108, 3128). Furthermore, 2-chlorohexadecanal was shown to induce COX-2 expression in human coronary artery endothelial cells (Messner MC et al., Lipids 2008, 43, 581). These data suggest that 2-chlorohexadecanal and possibly its metabolite 2-chlorohexadecanoic acid, both produced during leukocyte activation, may alter vascular endothelial cell function by upregulation of COX-2 expression. Thus,
2-chlorohexadecanal is not simply a lipid breakdown product but acts mainly as a signalling molecule of infection itself. Its presence indicates a site of infection or inflammation and attracts other neutrophils to the site.
Long after the demonstration of the presence of iodinated lipids in thyroid (besides iodinated aminoacids), it was shown that the major iodinated lipid formed in thyroid when incubated in vitro with iodide was 2-iodohexadecanal (Pereira A et al, J Biol Chem 1990, 265, 17018). In rat and dog thyroid, 2-iodooctadecanal was determined to be more abundant that the 16-carbon aldehyde. These compounds, which are thought to play a role in the regulation of thyroid function, were recently shown to be formed by the attack of reactive iodine on the vinyl ether group of PE plasmalogen. This attack generates an unstable iodinated derivative which breaks into lysophosphatidylethanolamine and 2-iodo aldehydes (Panneels V et al, J Biol Chem 1996, 271, 23006).
In some bacteria, aldehyde analogs of cyclopropane fatty acids were described.
Several fatty aldehydes are known to have pheromone functions. Studies in African and Asian countries have shown that the use of 10,12-hexadecadienal could be effective for control of the spiny bollworm Earias insulana, a cotton pest. The sex pheromone of the navel orange worm, Amyelois transitella, 11,13-hexadecadienal, is usually used in the control of this citric pest.
A branched saturated aldehyde (3,5,9-trimethyldodecenal, stylopsal) has been identified as a female-produced sex pheromone in Stylops (Strepsiptera), an entomophagous endoparasitic insect (Cvacka J et al., J Chem Ecol 2012, 38, 1483).
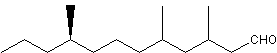
Stylopsal
Several isoprenoid aldehydes are important in insect biology as pheromones and in botany as volatile odorous substances. Some examples are given below:
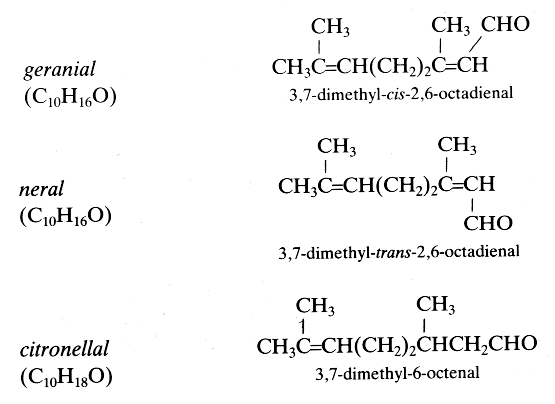
These three terpenic aldehydes are produced in large amounts by the mandibular glands of ants and may function as defensive repellents (Regnier FE et al., J Insect Physiol 1968, 14, 955). In contrast, the same molecules have a role of recruiting pheromones in honeybees. Citronellal or rhodinal is the main component in compounds having lemon scent as citronella oil. Citronellal has insect repellent properties, and appears highly efficient against mosquitoes but is also used for its aromatic properties in parfumary, cosmetics, candles and soaps.
Citral, a mixture of the tautomers geranial (trans-citral) and neral (cis-citral) is a major component (more than 60%) of the lemongrass (Cymbopogon flexuosus) oil. Lemongrass is widely used, particularly in Southeast Asia and Brazil, as a food flavoring, as a perfume, and for its medicinal properties (analgesic and anti-inflammatory). It was found that citral is a major suppressor of COX-2 expression and an activator of PPAR a and g (Katsukawa M et al., Biochim Biophys Acta 2010, 1801, 1214)
It was demonstrated that damaged leaves released 2-hexenal, among other C6-volatile aldehydes, produced from the catalytic activity of hydroperoxide lyase (Turlings TC et al., Proc Ntl Acad Sci USA 1995, 92, 4169). These compounds, considered as signal molecules, can trigger several responses in neighboring plants and may also act as antimicrobial agents (Farmer EE, Nature 2001, 411, 854).
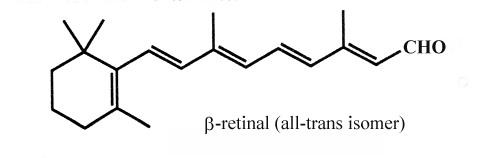
One important constituent of this group of aldehydes is retinal, one active form of vitamin A involved in the light reception of animal eyes but also in bacteria as a component of the proton pump.
 .
.
Retinal exist in two forms, a cis and a trans isomer. On illumination with white light, the visual pigment, rhodopsin is converted to a mixture of a protein (opsin) and trans-retinal. This isomer must be transformed into the cis form by retinal isomerase before it combines again with opsin (dark phase). Both isomers can be reduced to retinol (vitamin A) by a NADH dependent alcohol dehydrogenase. Retinol is stocked in retina mainly in an acylated form.
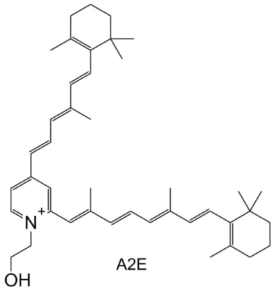
Sometimes, the retinal escapes this cycle and reacts with the ethanolamine headgroup of phosphatidylethanolamines. More rarely, in association with exposure to bright light, a molecule of retinal can react with another molecule of retinal and one loe of ethanolamine to form N-retinylidene-N-retinylethanolamine (A2E). It can accumulate in the eye and is associated with age-related eye diseases such as macular degeneration. It’s chemical identity was elucidated in 1996 (Sakai N et al., J Am Chem Soc 1996, 117, 1559). While A2E formation is rare, there is no enzymatic mechanism to degrade it. Over time, this orange-coloured and fluorescent molecule accumulates in retinal pigment epithelial cells in fluorescent granules called lipofuscin.
Cinnamic aldehyde (cinnamaldehyde) is the key flavor compound in cinnamon essential oil extracted from Cinnamomum zeylanicum and Cinnamomum cassia bark. Investigations have revealed than that benzyl aldehyde activates the Nrf2-dependent antioxidant response in human epithelial colon cells (Wondrak GT et al., Molecules 2010, 15, 3338). Cinnamic aldehyde may therefore represent a precious chemopreventive dietary factor targeting colorectal carcinogenesis.
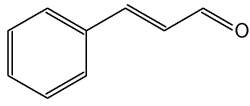
Cinnamic aldehyde
Safranal is a lipids compound (monoterpene aldehyde) isolated from saffron, the spice consisting of the stigmas of crocus flowers (Crocus sativus) and which is the main constituent responsible for the aroma of saffron. It likely originates by degradation of the carotenoid zeaxanthin. Several studies have shown several pharmacological activities of safranal including anti-oxidant, anti-inflammatory, cardioprotective, neuroprotective, nephroprotective, gastrointestinal protective. According to data published so far, safranal has the potential to exert several protective effects in neurological disorders (Fazeli E et al., Curr Mol Med 2023, 23, 952).
A study was designed to review these pharmacological and medical effects (Esmaealzadeh D et al., Iran J Basic Med Sci 2023, 26, 1131).
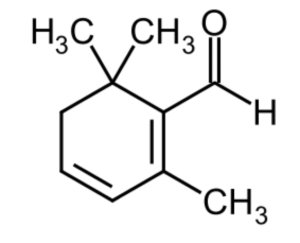 Safranal
Safranal
The native compound responsible for saffron’s pungent flavour is not safranal but picrocrocin which is the product of a union of safranal and a carbohydrate. That compound has insecticidal and pesticidal properties, and may comprise up to 4% of dry saffron. When saffron is dried after harvest, enzymatic actions split picrocrocin into D–glucose and a free safranal molecule responsible of saffron distinctive aroma. Safranal may comprise up to 70% of dry saffron’s volatile fraction. Chemists have found that a second molecule (lanierone or 2-hydroxy-4,4,6-trimethyl-2,5-cyclohexadien-1-one) is the most powerful contributor to saffron’s fragrance, despite its presence in a lesser quantity than safranal.
Safranal has an anticonvulsant effect in animal models, it acts also as an agonist at GABAA receptors and exhibits high antioxidant and free radical scavenging activity. with cytotoxicity towards cancer cells in vitro (Escribano J et al., Cancer Lett 1996, 100, 23).
Devenez membre et participez au développement de la Lipidomique au XXIème siècle.
S'inscrire