
Cyclic hydrocarbons, also named ARENES) are typically split into two categories :
1 – BENZOIDS that contain a benzene derivative and follow the benzene ring model,
2 – NON-BENZOIDS that contain other cycle derivatives,
3 – HETEROARENES when they contain a furan, a pyridine or another group.
Several chemical compounds derived from petroleum distillation and refining belong to what is named “mineral oil aromatic hydrocarbons”. Among them, some can enter food in many ways (environment, lubricants, food additives, migration from contact materials). Some of them may be genotoxic and thus they can damage DNA in cells and may cause cancer.
A1 – BENZOIDS
These hydrocarbons may contain only one cycle (Monocyclic benzoids), two cycles (Bicyclic benzoids) or three cycles (tricyclic benzoids).
A1.1 – MONOCYCLIC BENZOIDS
Several branched-alkylbenzenes have been described in Archaebacteria such as Thermoplasma and Sulfolobus. They have mostly two methyl groups branched on a saturated chain of 9 to 12 carbon atoms. One of them is shown below.
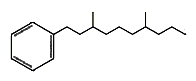
Long chain alkyl benzenes and alkyl toluenes are common con- stituents of crude oils and sedimentary organic matter. Among them, phytanyl benzene (and phytanyl toluene) occurs in mudstones from Permian-Triassic Boundary sections from Spitsberg and Greenland), as well as Western Australia. Despite their ambiguous origin, the differences in their distributions indicate that they can be characteristic of specific microbial communities such as Archaea (Grotheer H et al., Org Geochem 2017, 104, 42).
Phytanylbenzene
Dehydrozingerone can be obtained from ginger (Zingiber officinale, Zingiberaceae) or through a laboratory-based synthesis procedure. It is known as a feruloylmethane analog of curcumin, but also as a metabolite that contains half of the structure of curcumin. It possesses antioxidant and anti-inflammatory activities that are similar to those of curcumin including a potential anti-prostate cancer inhibitor (Mapoung S et al., Molecules 2020, 25(12), 2737). Results indicated that dehydrozingerone possessed a strong neuroprotective effect through its ability to ameliorate the Parkinson’s disease-like phenotypes in Drosophila model (Setzu MD et al., Biomolecules 2024, 14(3), 273). If validated in mammalian models, it could be considered a promising compound for the development of agents with potent neuroprotective properties.
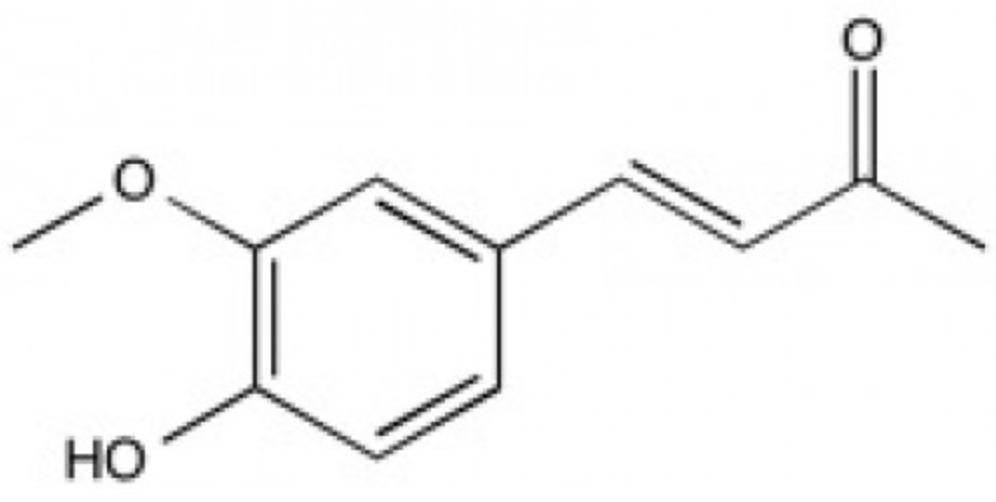 Dehydrozingerone
Dehydrozingerone
Cuminaldehyde or cuminal (4-isopropylbenzaldehyde) is a natural compound with an isopropyl group substituted in the 4-position. It is a constituent of the essential oils of eucalyptus, myrrh, cassia, and mainly cumin (Cuminum cyminum). Cumin (Cuminum cyminum), an aromatic plant belonging to the Apiaceae family, is cultivated in a region extending from the Eastern Mediterranean to India and his seeds are widely used as a spice.
It has a pleasant smell and is used commercially in perfumes and other cosmetics. Cuminaldehyde has shown promise due to its potential effects on antimicrobial, inflammation, and oxidative stress (Yilmaz E et al., Anim Biotechnol 2023, 34, 2766). Findings have suggested that cuminaldehyde may represent a potential alternative therapeutic agent for colitis (Yilmaz E et al., Food Biosci 2025, 71, 107284).
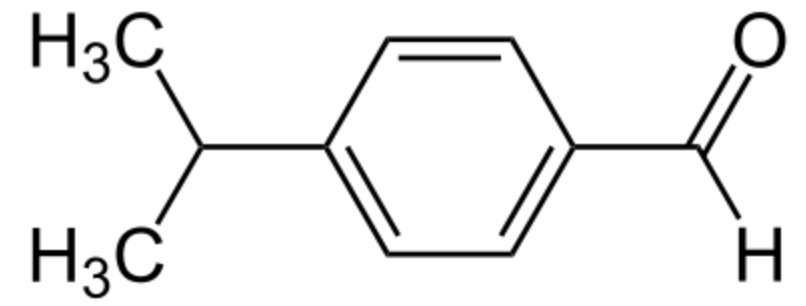
Cuminaldehyde (cuminal)
2-Phenylethanol (or phenethyl alcohol) is a hydrocarbon occuring widely in nature, largely in a variety of essential oils. It is found in extract of rose, carnation, hyacinth, Pinus halepensis, orange blossom, ylang-ylang, geranium, and neroli. It is therefore a common ingredient in flavors and cosmetics, when the odor of rose is desired. It is industrially prepared through the Friedel-Crafts reaction between benzene and ethylene oxide in the presence of aluminium trichloride. 1-Phenylethanol is a chiral isomer of 1-phenylethanol.
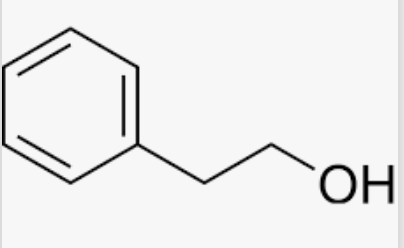
2-Phenylethanol
Phthalates are esters of phthalic acid with different alcohols (methyl, isobutyl, butyl, hexyl, octyl …). The most important form is di-(2-ethylhexyl)-phtalate (DEHP), which accounts for about 50% of the world production of phthalates (Wikipedia) which are used as plasticizers.
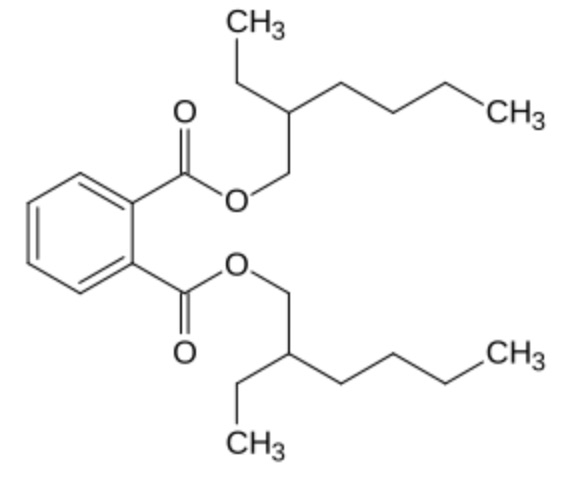
Di-(2-ethylhexyl)-phtalate
Phthalates are mainly used in cosmetics (perfume, hair spray, soap, shampoo …) and in a lot of consumer products made in various plastic substances, therefore they are commonly found in fat-containing foods despite the international decision that they can only be used as plasticizers for plastic material in contact with non-fatty food. Their biodegradation in soil occurs by sequential hydrolysis of the two diethyl chains but that reaction occurs very slowly in an abiotic environment. Studies have suggested that some phthalates affect male reproductive development via inhibition of androgen biosynthesis but it seems more evident that certain high doses of phthalates may cause skeletal malformations after administration in pregnant animals,
A benzyl cyanide derivative has been described in the butterfly Pieris brassicae ( Andersson J et al., J Chem Ecol 2003, 29, 1489). It functions as an anti-aphrodisiac transferred by the male to the female.
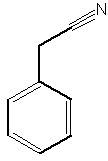
Benzyl cyanide
Phenylethyl isothiocyanate, one of the most important isothiocyanates (R-N=C=S), is present in the cruciferous vegetable watercress (Nasturtium officinale). It is the most potent antioxidant among Brassicaceae, present as glucosinolate gluconasturtiin, which, on hydrolysis, produces phenylethyl isothiocyanate. This biosynthesis takes part in the formation of “mustard oil bomb“, a chemical defense system against herbivory found in members of the cabbage family.
Phenylethyl isothiocyanate exhibits antioxidant and anti-inflammatory, bactericidal), and anti-carcinogenic effects, among other benefits. Recent reviews suggest that it could be a natural nutraceutical or an adjuvant to treat various disorders due to its chemopreventive action and health-promoting effects (Li N et al., Food Chem 2022, 375, 131816).
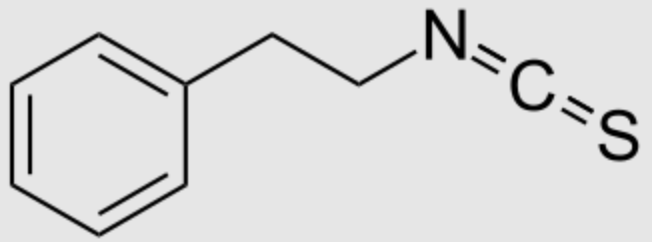
Phenylethyl isothiocyanate
It has been demonstrated that solitary and gregarious locusts are attracted by 4-vinylanisole (or 4-methoxystyrene). Furthermore, 4-vinylanisole could function to both bring solitary locusts into the fold of the swarm, as well as maintain a swarm’s cohesiveness over time (Guo X et al., Nature 2020). The finding could inform new ways of controlling or preventing locust swarms, potentially by attracting the insects with their own scents.
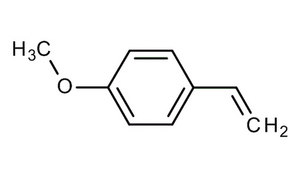 4-Vinylanisole
4-Vinylanisole
Bibenzoyl compounds are present in various plant species and mainly in liverworts, a group of non-vascular land plants forming the division Marchantiophyta (hepatics). These bibenzoyls (two aromatic rings) and bisbibenzyls (four aromatic rings), are now considered as unique signature molecules of these plants (Sen K et al., Plants 2023, 12, 4173).
3-Methoxybibenzyl has been described as a major component of Radula complanata (Hepaticae) oil. It appears to be responsible of an allergenic contact dermatitis (Asakawa Y et al., 1978, 17, 2115).
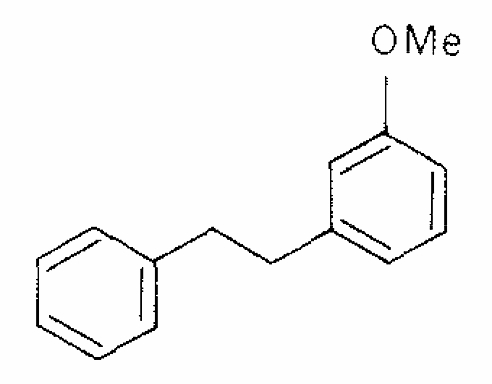 3-Methoxybibenzyl
3-Methoxybibenzyl
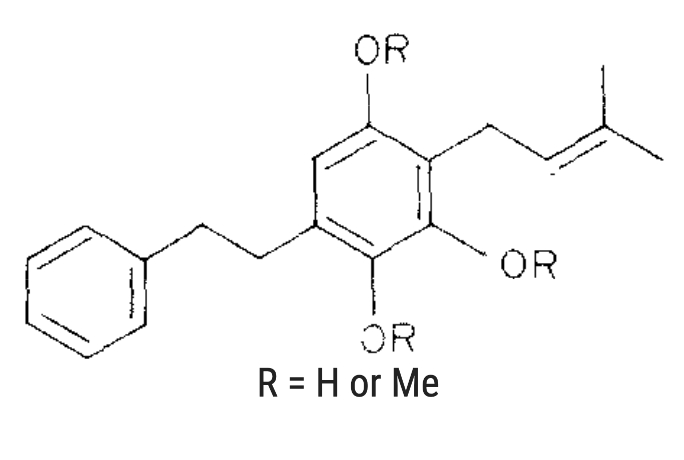
Several prenyl bibenzyl derivatives have been isolated from four Radula species. Two of them are given below (Asakawa Y et al., Phytochemistry 1982, 23, 2481).
The stilbenes (or stilbenoids) form a group of numerous compounds classified in the phenylpropanoids, based on a simple one, stilbene. That compound consists of two phenyl groups linked by a trans ethene double bond. Its name was derived from the Greek word “stilbos”, which means shining. Stilbene is mainly used in manufacture of dyes and optical brightening agents.
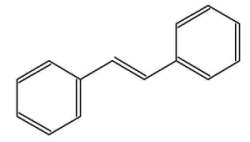
trans-Stilbene
Stilbenes, sometimes classed into the polyphenol group, are present in several vegetal sources. Several forms have been described, differentiated by various substitutions and combinations of hydroxyl or alkoxyl groups. Some stilbenes are glycosylated. Thus, 2,3,5,4′-tetrahydroxystilbene 2-O-b-D-glucoside, the major bioactive compound from Polygonum multiforum, can efficiently inhibit the formation of advanced glycation end products (Lv L et al., J Agric Food Chem 2010, 58, 2239). A comprehensive review of the source plants, chemistry, biosynthesis, pharmacology, application of stilbenes have been reviewed (Teka T et al., Phytochemistry 2022, 197, 113128).
Studies on the phytochemical composition of different species of rhubarb, Rheum genus (Polygonaceae), were undertaken as early as at the beginning of the 20th century, and provided information on the presence of a variety of stilbenes (review in Kolodziejczyk-Czepas J et al., Phytochem Rev 2021, 20, 589).
A review of the chemistry and the biology of natural stilbenes may be consulted (Zhou L et al., Nat Prod Rep 2025, 42, 359). A review focused to selected bioactive stilbenes as potential chemopreventive agents for colorectal cancer with a focus on their modulatory mechanisms of action, especially in targeting alterations in DNA methylation machinery (Fialková V et al., Nutr Cancer 2024, 76, 760).
Stilbenes belong to the naturally synthesized plant phytoalexins, produced de novo in response to various biotic and abiotic stressors. The importance of stilbenes in plant resistance to stress and disease is of increasing interest (review in Gao Q et al., J Agric Food Chem 2024, 72, 7655).
Diethylstilbestrol, a stilbene derivative, (E)-11,12-diethyl-4,13-stilbenediol, is also classified in the phenylpropanoids group. It is a nonsteroidal estrogen which was used for a variety of indications, including pregnancy support for women with a history of recurrent miscarriage, hormone therapy for menopausal symptoms and estrogen deficiency in women. Today, it is only used in the treatment of prostate cancer and less commonly breast cancer. It has been demonstrated it has the potential to cause a variety of significant adverse medical complications, including transgenerational effects, and thus it was largely discontinued.
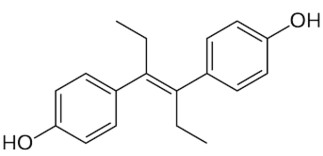 Diethylstilbestrol
Diethylstilbestrol
Resveratrol (3,4′,5-trihydroxystilbene), which belongs to the group of phenylpropanoids, is the most studied because of its presence in grapes and wine and some berries (blueberries, Vaccinium) and its potential pharmacological properties (anti-cancer, antiviral, neuroprotective, anti-aging, and anti-inflammatory). To date, there is no high-quality evidence that resveratrol improves lifespan or has a substantial effect on any human disease (wikipedia).
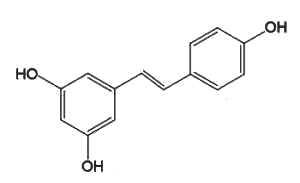
Resveratrol
Some studies have suggested that resveratrol has therapeutic properties in mental disorders, such as major depressive disorder, bipolar disorder, Alzheimer’s disease, and autism (Menegas S et al., J Nutr Biochem 2023, 121, 109435). A systematic review providing an unbiased conclusion on the therapeutic effectiveness of resveratrol in Alzheimer’s disease may be consulted (Azargoonjahromi A et al., Nutr Metabol 2024, 21). As it has been reported, the protective benefits of resveratrol seems to be in preventing the risk of Alzheimer’s disease induced by proinflammatory agents (Bartra C et al., Antioxidants 2024, 13, 177). Results in rats were obtained proving that resveratrol has a positive effect on the development of early retinal neurodegeneration in diabetic retinopathy (Wan Z-W et al., Eur J Nutr 2025, 64, 232).
It has been demonstrated that short chain fatty acid esters enhanced the stability and bioactivity of resveratrol and could be used as a functional food ingredient in processed foods or as dietary supplements to promote health (Huang YN et al., Nutrients 2024, 16, 4076). The effects of substitution sites and acyl chain length on antioxidant capacity and bioaccessibility of high-active resveratrol monoesters in vitro have been examined (Li D et al., Food Chem 2025, 480, 143845).
It has been determined that resveratrol is used by plants as a defensive element (they are named phytoalexins). Thus, grape vine attacked by mildew is able to product resveratrol which can be transformed into glycosylated or dimer compounds. The resistance of some cultivars seems to be related to the toxicity of the derivative (Pezet R et al., Physiol Mol Plant Pathol 2004, 65, 269). Similar observations were reported for conifers.
A resveratrol derivative, oxyresveratrol (4 hydroxy groups instead of 3) is found in the heartwood of Artocarpus lakoocha and in the traditional drug ‘Puag-Haad’ made from it. Interestingly, this compound was found to enhance the anti-cancer effect of cisplatin against epithelial ovarian cancer cells (Thaklaewphan P et al., Biomolecules 2024, 14(9), 1140).
It has been demonstrated that resveratrol monoesters, synthesized by esterifying with lipophilic groups, excel in lipophilicity, antioxidant activity, and bioavailability (Li D et al., Food Chem 15 March 2025, 143845).
Hopeaphenol is a resveratrol tetramer. It has been first isolated from Dipterocarpaceae like Shorea ovalis. It has also been isolated from some wines. It has been found that it exhibits cardioprotection-related activities, including anti-apoptosis and mitochondrial function regulation, and displays functional differences from other resveratrol derivatives (Seya K et al., J. Pharmacol Exp Ther 2009, 328, 90). Findings have identified hopeaphenol as a potential candidate for further development in the prevention and treatment of heart failure (Chen J et al., Nutrients 2025, 17, 3025).
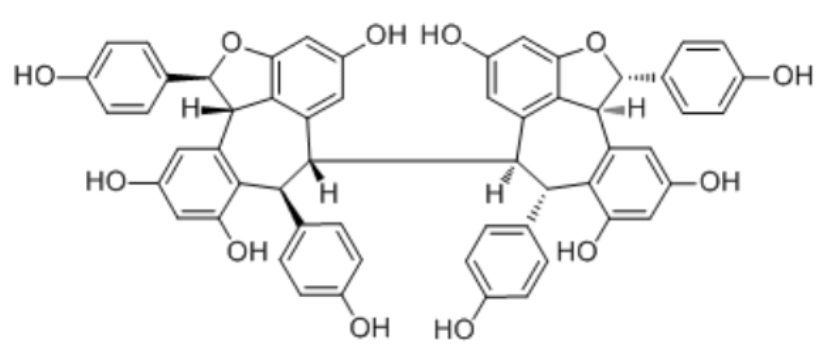 Hopeaphenol
Hopeaphenol
Pterostilbene is a stilbenoid chemically related to resveratrol. In plants, it serves a defensive phytoalexin role. It differs from resveratrol by exhibiting increased bioavailability due to the presence of two methoxy groups (increased lipophilic properties). Studies have reported dose-based elevations of low density lipoprotein cholesterol and decreased high density lipoprotein cholesterol. Pterostilbene was evaluated for its protective effects and safety during prolonged use on osteoarthritis (Lee YC et al. J Agric Food Che. 2024, 72, 9150).
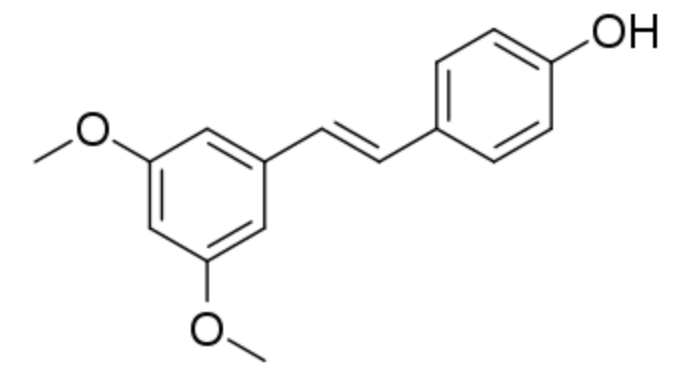 Pterostilbene
Pterostilbene
Dihydro-resveratrol is a dihydrostilbenoid found in wine. It is a metabolite of trans-resveratrol formed in the intestine by the hydrogenation of the double bond of the ethyl group by microflora.
Several more complex derimatives of resveratrol are of biochemical and physiological importance:
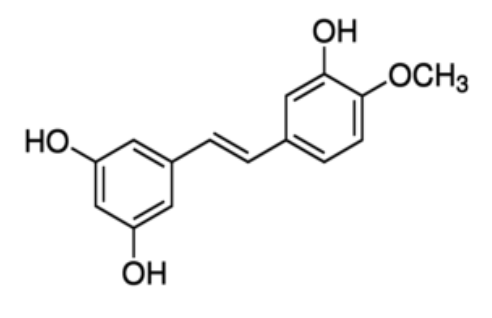
 Pterostilbene
Pterostilbene
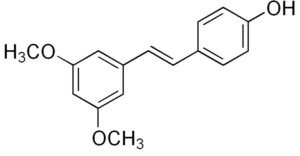
 Rhapontigenin
Rhapontigenin
Pharmaceutic industries have developed stilbene derivatives which have estrogenic activity (non-steroidal estrogens) such as diethyl stilbestrol.
Oligostilbenoids (oligo- or polystilbenes) are oligomeric forms of stilbenoids. Some molecules are large enough to be considered polyphenols and constitute a class of tannins (Wikipedia). These lipids, found in Paeonia seeds, have been associated with multiple biological activities (Li XJ et al., 11 Jun 2025 PLOS ONE).
Chalcone is an α,β-unsaturated ketone chemically named (2E)-1,3-Diphenylprop-2-en-1-one. Similar compounds are known collectively as chalcones or chalconoids (Wikipedia). They are widely known bioactive substances, fluorescent materials, and chemical intermediates. They are synthesized in plants as secondary metabolites. Dihydrochalcone is the reduced derivative of chalcone. There are several natural hydrochalcones (aspalathin, naringin dihydrochalcone, nothofagin, phloretin), and some derivatives have attracted attention as drugs. Phloretin is known to inhibit active transport of glucose into cells but that inhibition is weaker than that measured using its glycoside phlorizin (Wikipedia).
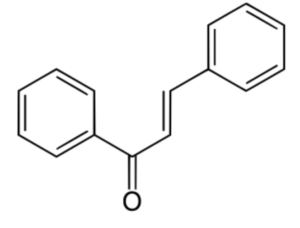 Chalcone
Chalcone
Fatty acid conjugated chalcones have been described as tubulin polymerization inhibitors, thus with potential antiproliferative activity (Mohamed SM et al., J nat Prod 2023, 86, 5, 1150). Decanoic acid conjugates were more potent than longer lipid analogues, with the most active being more potent than the reference tubulin inhibitor, combretastatin-A4 and the anticancer drug, doxorubicin.
Hispidin (6-(3′,4′-dihydroxystyryl)-4-hydroxy-2-pyrone) has been isolated in 1889 from the mushroom Inonotus hispidus (formerly Polyporus hispidus) as a naturally occurring styrylpyrone. Hispidin is a precursor of fungal luciferin, a compound responsible for light emission by luminous mushrooms. Later, a number of other styrylpyrone metabolites from Phellinus and Inonotus have been discovered. Styrylpyrone pigments are common constituents of fungi, mainly those belonging to the Hymenochaetaceae family, including Phellinus and Inonotus (Fiasson J L, Biochem Syst Ecol 1982, 10, 289). They also exist in primitive angiosperm families, including Piperaceae, Lauraceae, Annonaceae, Ranuculaceae and Zingiberaceae, where they have an important role in defense against mechanical wounding or microbial attack. and show potent biological activities, including anti-cancer and sedative effects. Styrylpyrone-class compounds from medicinal fungi Phellinus and Inonotus spp., and their medicinal importance have been reviewed (Lee K et al., J Antibiotics 2011, 64, 349).
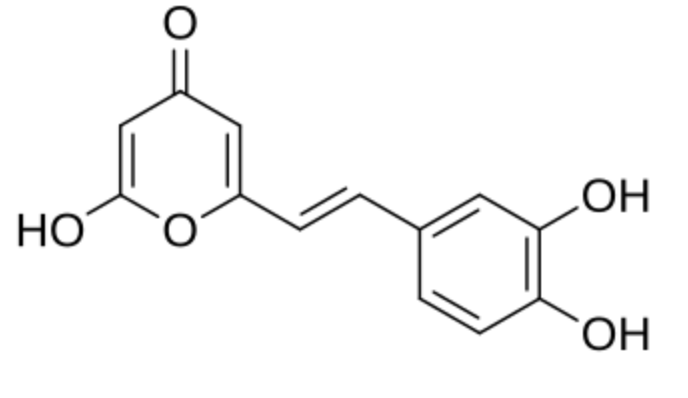 Hispidin
Hispidin
Diarylheptanoids belong to a compound group having phenyl rings at 1,7 positions of n-heptane, such as curcumin and several similar analogues found in the rhizomes of the ginger (Curcuma longa) family (Li J et al., J Nat Prod 2010, 73, 1667). Diarylheptanoids are class of compounds with limited distribution in plant kingdom, found mainly in families Zingiberaceae and Betulaceae.
Their common structure is shown below.
Diarylheptanoid
Curcumin is the principal diarylheptanoid of the Indian spice turmeric, which is a member of the ginger family (Zingiberaceae).
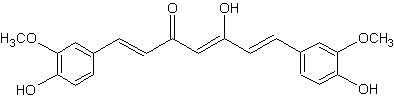
Curcumin
That pigment, which gives the yellow color to turmeric (E100), was isolated two centuries ago, and its structure as diferuloylmethane was determined in 1910 almost hundred years after it was first isolated in 1815 (Milobedzka J et al., Ber Deu. Chem Ges 1910, 43, 2163). Numerous therapeutic activities have been assigned to turmeric for a wide variety of diseases and conditions, they are likely partly related to its strong antioxidant properties (Aggarwal BB et al., Adv Exp Med Biol 2007, 595, 1). An extensive review has been published on the various properties of curcuminoids (Mukherjee S et al., in Herbs and Spices – New Processing Technologies, 2021, Rabia Shabir Ahmad Eds). Curcumin has been shown to inhibit gastric cancer cells through various mechanisms. A review summarized the mechanisms through which curcumin affects gastric cancer in both laboratory and animal studies (Hao M et al., J Funct Foods 2025, 125, 106677).
Plants of the Alnus genus (Betulaceae) have been found to be a valuable source of diarylheptanoids similar to curcumin but with various substituted chemical groups. Some of these compounds have valuable activities against LPS-induced inflammation and could be the source of new anti-inflammatory drugs (Lai YC et al., Phytochemistry 2012, 73, 84).
The reductive metabolites of curcuminoids (tetrahydrocurcuminoids) retain essentially all the useful health-promoting activities of curcuminoids and Japanese researchers have shown that they are more bioavailable than parent curcuminoids (Okada K et al., J Nutr 2001, 131, 2090). These derivatives are found in the plant kingdom, and arise from human metabolism of curcuminoids, but also result from gut microbiota transformation of curcumin. Tetrahydrocurcuminoids are produced chemically by hydrogenation of curcuminoids extracted from the rhizomes of C. longa. The European Food Safety Authority (EFSA) has approved the use of Sabinsa’s C3 Reduct®, a mixture of the most prominent reduced metabolites, for the target population at 140 mg/day.
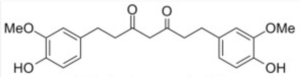 Tetrahydrocurcumin
Tetrahydrocurcumin
Polybrominated biphenyl ethers such as 2-(2′,4′-dibromophenyl)-4,6-dibromophenol are characteristic secondary metabolites of the dictyoceratid marine sponge Dysidea herbacea, common in shallow waters of the tropical Pacific Ocean and may represent as much as 12% of the dry weight (Unson MD et al., Marine Biology (1994) 119:1). The production of that compound was shown to be due to the cyanobacterium, and not to the sponge or symbiotic heterotrophic bacteria.
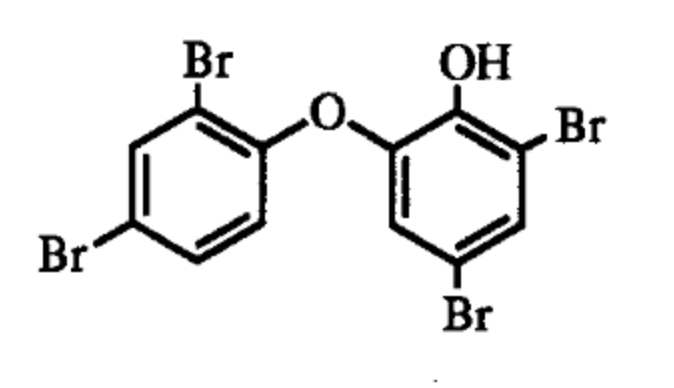 One of the Dysidea polybrominated biphenyl ethers
One of the Dysidea polybrominated biphenyl ethers
It was reported that similar compounds present in the shallow water sponge Lamellodysidea herbacea are active as chemical defense against fish feeding and environmental relevant bacteria, especially pathogenic bacteria. These properties might be one reason for the widespread occurrence of that sponge in Indonesia and the Indo-Pacific (Faisal MR et al., Mar Drugs 2021, 19(11), 611).
Cannabidiol (CBD) or 2-[(1R,6R)-6-isopropényl-3-méthylcyclohex-2-én-1-yl]-5-pentylbenzène-1,3-diol is the first phytocannabinoid discovered in 1940 in hemp (Cannabis sativa). It is one of more than 110 similar compounds (cannabinoids) in that plant. Despite numerous clinical studies, CBD is used as dietary supplement but with unproven claims of precise therapeutic effects (Wikipedia).
The lack of fundamental knowledge about some scientific aspects of CBD and of parent molecules stems from a worldwide prohibition (Duggan PJ, Aust J Chem 2021, 74, 369).
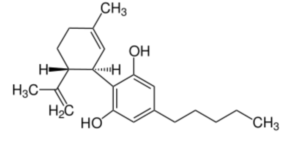 Cannabidiol (CBD)
Cannabidiol (CBD)
A derivative of cannabidiol, Tetrahydrocannabinol (THC), is the principal psychoactive constituent of cannabis. Despite the presence of multiple isomers, the term THC refers to the Delta-9-THC isomer with the chemical name (−)-trans-Δ9-tetrahydrocannabinol, a molecule related to terpenes. Investigations have demonstrated that, when smoked, THC is absorbed into the bloodstream and reaches the brain, combining with endocannabinoid receptors located in the cerebral cortex, cerebellum, and basal ganglia, parts of the brain responsible for thinking, memory, pleasure, coordination and movement.
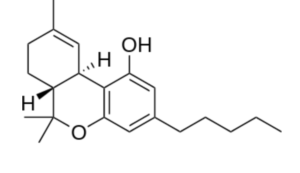 Tetrahydrocannabinol (THC)
Tetrahydrocannabinol (THC)
During investigation of natural products in marine microorganisms, 2,4,6-triphenyl-1-hexene was isolated from a marine-derived Bacillus sp. strain called APmarine135 (Kim HY et al., Mar Drugs 2024, 22(2), 72). In vitro investigations have suggested that this component is a promising anti-melanogenic agent in the cosmetic industry. This lipid has been previously isolated from a fungus Phellinus pini and a marine bacteria Solwaraspora sp. It has been also determined that it exhibited estrogenic activity in MCF-7 cells.
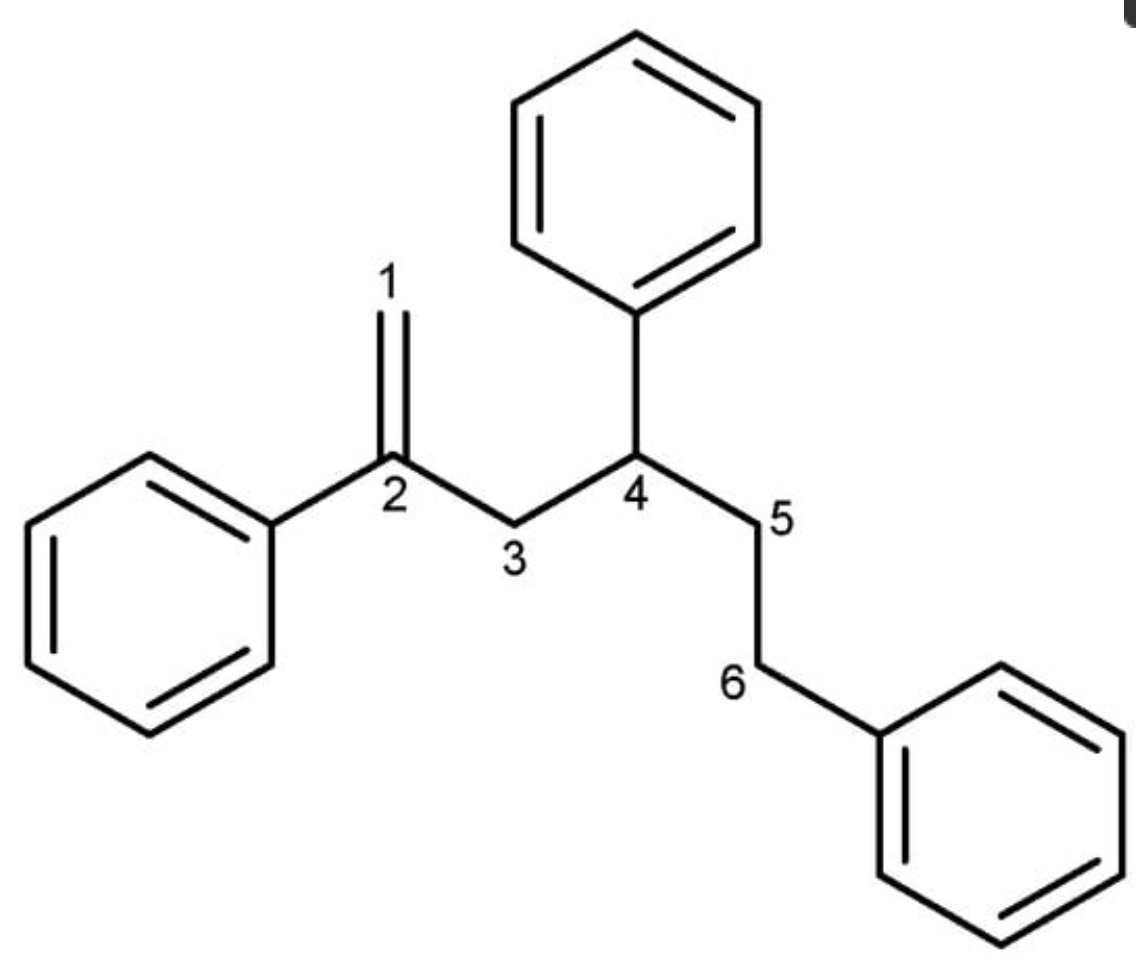
2,4,6-Triphenyl-1-hexene
Clemastine (also known as meclastin) is an oral antihistamine that works by promoting the development of myelin-making cells, called oligodendrocytes (Wikipedia). An important trialled to considered that Clemastine administration may be a remyelinating therapy for multiple sclerosis (Green AJ et al., The Lancet 2017, 390, 10111, 2481) but clinical trial was halted in early 2024 due to important side effects in some patients.
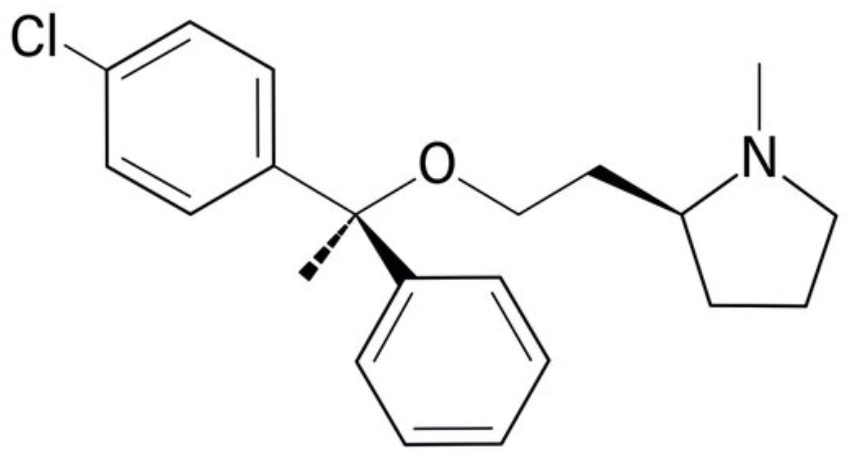 Clemastine
Clemastine
A2 – NON-BENZOIDS (other aromatic cyclic derivatives)
A2.1 – MOCYCLIC NON-BENZOIDS
One of the simplest monocyclic non-benzoids is 1-Methylcyclopropene, a synthetic derivative of cyclopropene used as a plant growth regulator. It turns to vapor at temperatures higher than 12°C. It is structurally related to the natural plant hormone ethylene and it is used commercially to slow down the ripening of fruit and to help maintain the freshness of cut flowers. Its mechanism of action involves its tightly binding to the ethylene receptor in plants, thereby blocking the effects of ethylene (ripening of climacteric fruit, opening of flowers, shedding of leaves). Its use has been approved and accepted for use in more than 34 countries including the European Union and the United States.
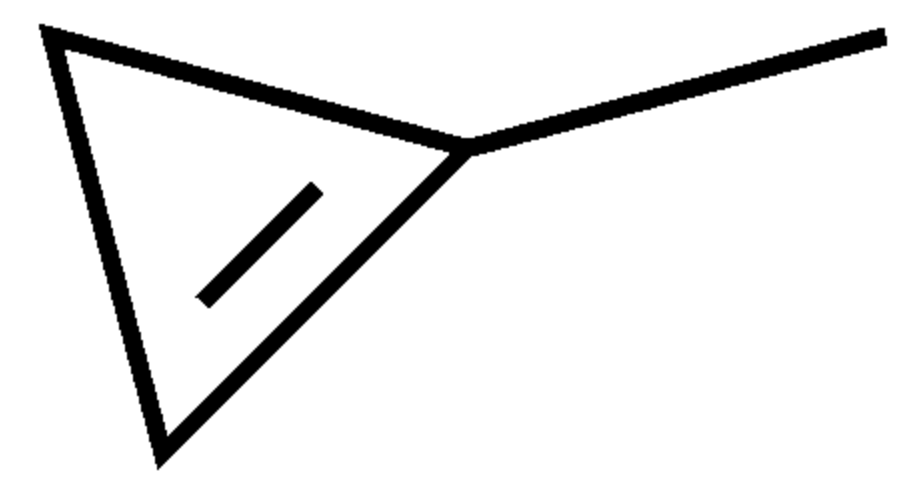 Methylcyclopropene
Methylcyclopropene
Among the numerous dictyocarpenes, dictyopterene A (trans-1-(trans-1-hexenyl)-2-vinylcyclopropane) is naturally present in marine and freshwater environments and in lipids extracted from several species of brown algae such as Dictyopteris (Phaeophyceae) (Jiittner F et al., Limnol Oceanogr 1984, 29, 1322). Dictyopteris is an important group of marine seaweeds and is widely distributed in tropical, subtropical and temperate regions. This genus is known by its richness in chemicals which are in part the source of its characteristic “ocean smell” (Zatelli GA et al., Revista Brasileira de Farmacognosia 2018, 28, 243).
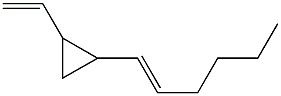 Dictyopterene A
Dictyopterene A
Sarekensane is the first nonterpenoid cuticular hydrocarbon from Collembola that is biosynthesized via the fatty acid pathway, as are insect hydrocarbons, and contains two cyclopropane rings in the chain, not previously reported from arthropods (Möllerke A et al., J Nat Prod 2024, 87, 85).
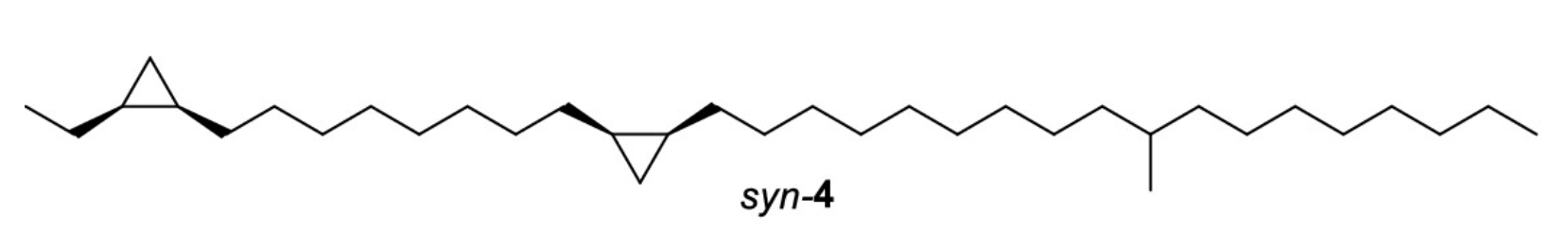 Sarekensane
Sarekensane
Ectocarpene – After the first hypothesis of a communication system (chemotaxis) by Thuret MG for the fertilization of brown algae, 117 years were needed to know the structure of the first algal pheromone (Müller DG et al., Science 1971, 171, 815). This compound, ectocarpene, is an unsaturated heptacyclic hydrocarbon found in the brown algae Ectocarpus, Adenocystis and Sphacelaria. It belongs to a large group of chemicals named dictyopterenes. That compound has a fruity scent and can be sensed by humans when the female organs start emitting the substance to attract the male gametes.
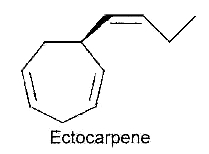
Several heptacyclic compounds similar to ectocarpene play a role of pheromone in marine brown algae, dityotene in Dictyota sp, desmarestene in Desmarestia sp and Cladostephus sp, lamoxirene in Laminaria sp, Alaria sp and many others.
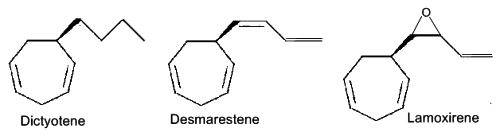
Heptacyclic compounds from brown algae
The C11-hydrocarbon ectocarpene was also detected as component in the odor of ripening mango (Berger RG et al., J Agric Food Chem 1985, 33, 232) but was also shown to be a metabolite in leaves of the Asteraceae Senecio isatideus (Bohlmann F et al., Phytochemistry 1979, 18, 79). Several biosynthetic studies suggest unsaturated fatty acids as precursors of these pheromone hydrocarbons (Pohnert G et al., Nat Prod Rep 2002, 19, 108). A review of hydrocarbon as chemical signals in algal gamete attraction has been released (Boland W, PNAS 1995, 92, 37).
Several parent molecules (linear, tri-, penta- or hexacyclic) with different degrees of unsaturation and chain lengths have been described in various algal species (Pohnert G et al., Nat Prod Rep 2002, 19, 108).
A2.2 – POLYCYCLIC NON-BENZOIDS
These hydrocarbons, whose only few groups are present in plants, contain fused rings containing only carbon, they are named Polycyclic Aromatic Hydrocarbons (PAH).
PAH consist of two or more fused aromatic rings: PAH with less than five aromatic rings are called “light PAH”, when five and more rings are present they are called “heavy PAH” (HAP). Most of the HAP are genotoxic, 8 of them being classified as a carcinogen of category 2. The European Commission (EC) regulation has decided a limit of 2 μg/kg, and 10 μg/kg limit for the sum of 4 PAH.
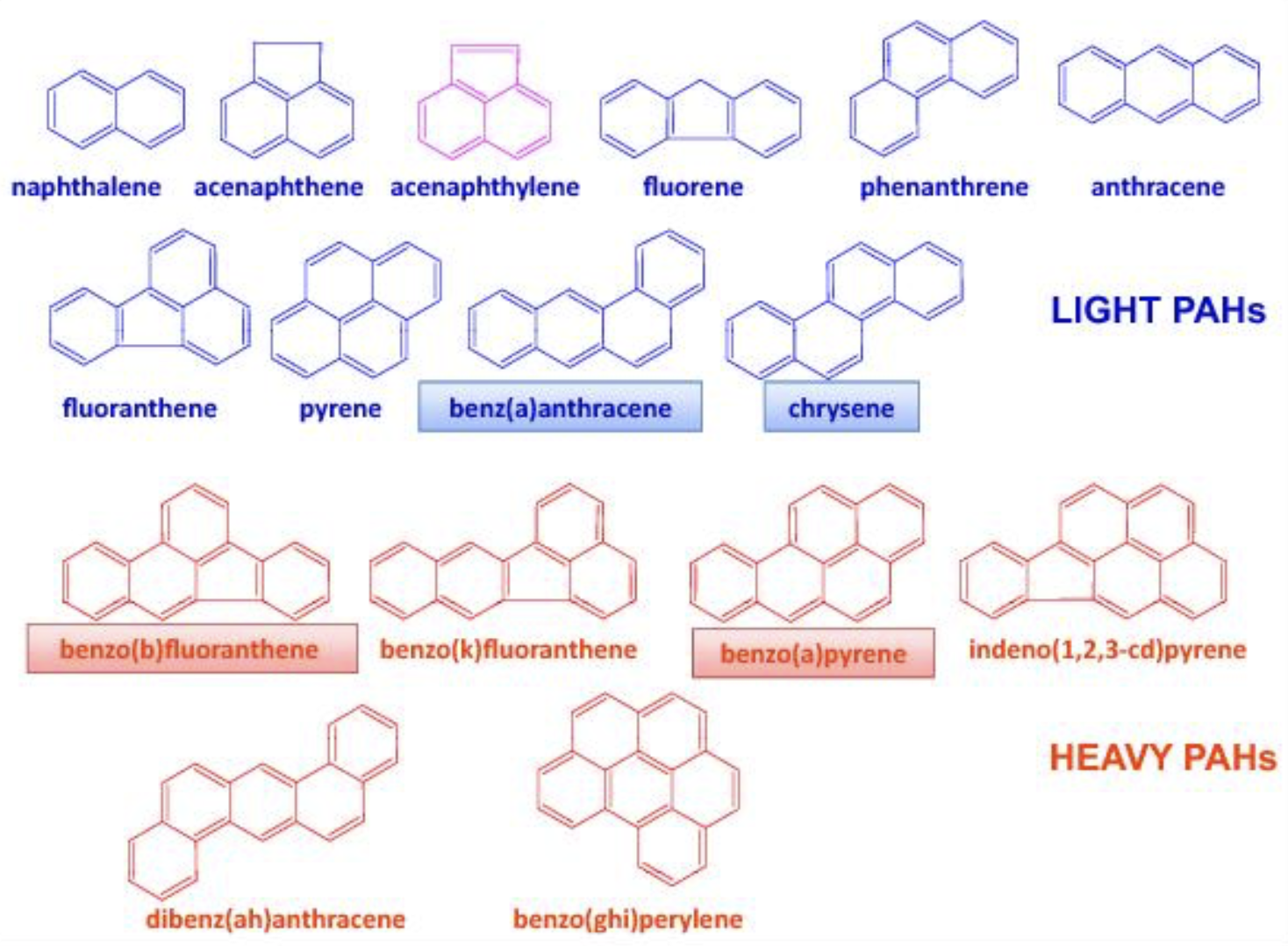
Structure of PAH accoding to Lacoste F (OCL 2014, 21, A103)
The great majjority of PAH found in living matters are formed by incomplete combustion or pyrolysis of organic material such as wood, petroleum products, coal or food. Thus, they are detected in different types of food particularly grilled meat, cereals, fats and oils. It has been several times demonstrated that barbecued foods produce carcinogenic polycyclic aromatic hydrocarbons such as benzo[a]anthracene, chrysene, benzo[b]fluoranthene, and benzo[a]pyrene due to incomplete charcoal combustion, direct food-fuel contact, and excessive heating (Wong S et al., J Food Comp Anal 2025,
137, Part A, 106890).
Their presence in vegetable oils (mainly coconut oil, pomace olive oil and sunflower oil) may result from the production process or environmental contamination. Light PAH are eliminated by the deodorization step while heavy ones are eliminated during the bleaching step.In insects.
Naphthalene is a constituent of Magnolia flowers (Azuma H et al., Phytochemistry 1996, 42, 999). It may function as protection of tissue against chewing insects and it may attract insects to pollinate by the UV absorption of accumulated naphthalene in the floral parts and floral scent. Naphthalene, playing a role of insect repellent, was shown to be produced by Muscodor vitigenus, a novel endophytic fungus (Daisy BH et al., Microbiology 2002, 148, 3737).
In insects, it has been found that naphthalene is present in extracts of the nest of Formosan subterranean termites. This is the first time naphthalene has been found naturally associated with any insect species (Chen J et al., Nature 1998, 392, 558).
Naphthalene
Polychlorinated naphthalene (PCN) are produced upon treatment of naphthalene with chlorine, they are a group of synthetic industrial chemicals with high thermal stability and inertness. Commercial PCNs are mixtures of up to 75 components and byproducts. PCNs were once used in insulating coatings for electrical wires, as well as other applications. PCNs are also unintentionally produced and released during high-temperature industrial processes, including waste incineration, cement production, and non-ferrous smelting. While some PCNs can be broken down by sunlight and by certain microorganisms, many PCNs persist in the environment.
Perylene is a typical polyaromatic hydrocarbon that occurs in many sediments. Possible precursors include binaphthyl compounds derived from fungi, as those cited above, in which case a high abundance of sedimentary perylene might indicate a moist and humid continental climate in the depositional environment (Suzuki N et al., Org Geochem 2010, 41, 234). Perylene has the chemical formula C20H12 and occurrs as a brown solid. It or its derivatives may be carcinogenic, and considered as a dangerous pollutant. Perylene is used as a fluorescent lipid probe for cell membrane cytochemistry.
Perylene
Several characteristic larger polycyclic aromatic hydrocarbons (heavy PAH) such as the eight-ring 1,2,3,4,5,6-hexahydrophenanthro[1,10,9,8-opqra]perylene (HHPP) have been isolated from fossil crinoids which contain phenanthroperylene quinone pigments. The diagenetic origin of these molecules has been studied in fossil crinoids (Wolkenstein K, Org Geochem 2019, 136:103892).
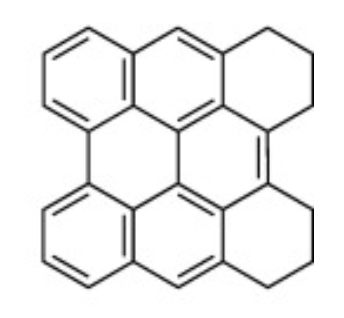 HHPP
HHPP
Diamondoids : they are variants of the carbon cage molecule known as adamantane (C10H16), the smallest unit cage structure of the diamond crystal lattice.
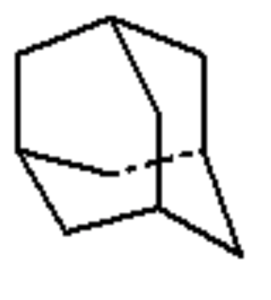 Adamantane
Adamantane
They may include one or more cages (adamantane, diamantane, triamantane, and higher polymantanes).
Diamondoids occur naturally in petroleum deposits and have been extracted and purified into large pure crystals of polymantane molecules having more than a dozen adamantane cages per molecule. It has been shown that the diamondoids found in petroleum (crude oil and condensates), coal and sedimentary rock in the geosphere and are considered to form during early diagenesis. They are composed of carbon from biological sources. These compounds are widely used to reflect thermal maturation of high mature source rocks or oils and oil cracking extents.
High concentrations of sulfated diamondoids (thiadiamondoids) together with elevated H2S are indicative of the occurrence and extent of thermochemical sulfate reduction.
Propellanes : Propellanes are polycyclic compounds in which three ring systems share a single bond. They form an exciting class of tricycloalkanes that covers a wide array of natural and synthetic products. The most studied natural product containing a propellane core is modhephene. This sesquiterpene was isolated in 1978 from Isocoma wrightii (Asteraceae) (Zalkow LH et al., J Chem Soc, Chem Commun 1978, 420). Since then, it was found in different plants of the Asteraceae family, such as in the roots of Silphium perfoliatum, in the rhizomes of Echinops giganteus and in many species of the Berkheya genus.
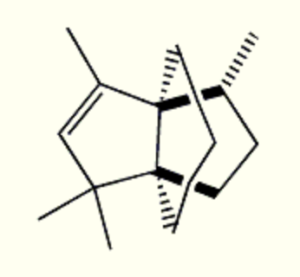 Modhaphene
Modhaphene
Several other natural products that have a [3.3.3]propellane core including modhephene derivatives were isolated from different plant sources. A review of the occurrence, synthesis and applications of natural and designed [3.3.3]propellanes may be consulted (Dilmaç AM et al., Nat Prod Rep 2020, 37, 224).
1-Naphthaleneacetic acid is a synthetic plant hormone in the auxin family and is an ingredient in many commercial horticultural products. it is a rooting agent and used for the vegetative propagation of plants from stem and leaf cuttings. It is also used for plant tissue culture (Wikipedia).
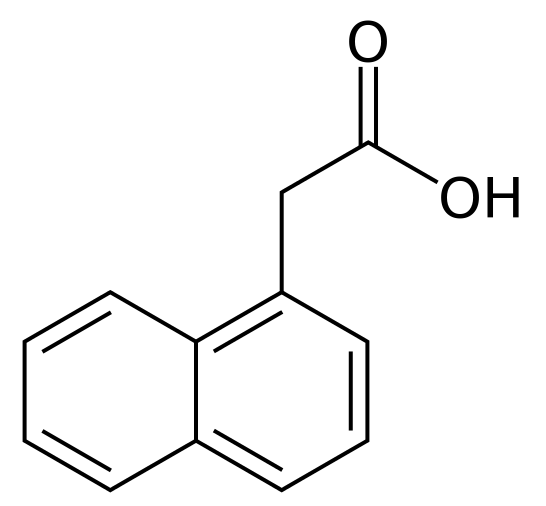 1-Naphthaleneacetic acid
1-Naphthaleneacetic acid
Naphthoquinones are present in the secretion of scent glands of Opiliones (Raspotnig G et al., J Chem Ecol 2010, 36, 158). The two main components which serve chemical defense in these animals are 1,4-naphthoquinone and its methylated derivative 6-methyl-1,4-naphthoquinone.
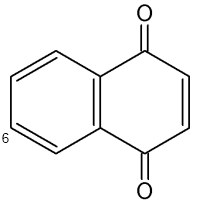
1,4-Naphthoquinone
Derivatives of 1,4-naphthoquinone were found in sea urchin coelomocytes (red spherule cells), mainly echinochrome A (or spinochrome A), (7(2)-ethyl-2, 3, 5, 6, 8-pentahydroxy 1, 4-naphthoquinone, known as one polyhydroxynaphthoquinone) which is located within cytoplasmic vesicles. Il can also be isolated from the urchin spines. Despite their identification over a century ago, and evidence of antiseptic properties, some progress has been made in characterising the immunocompetence properties of that product. It is the most studied molecule of this family and is an active principle approved to be used in humans, usually for cardiopathies and glaucoma. It is used as the active ingredient of the commercially available drug Histochrome® (Mishchenko NP.et al., Pharm Chem J 2003, 37, 48). as a cardioprotective and antioxidant drug. It is also used in ophthalmology for the treatment of macular degeneration, cornea and retina degenerative diseases. It was found that co-treatment with echinochrome A is able to prevent this decrease in membrane potential and increase in ROS level (Jeong SH et al., Marine Drugs 2014, 12, 2922).
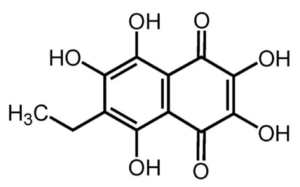 Echinochrome A
Echinochrome A
Most importantly, it has been shown that echinochrome A contributes to the modulation of the immune system. It can regulate the generation of regulatory T cells and inhibit pro-inflammatory cytokine production, These mechanisms suggest that it could be a candidate drug to alleviate the cytokine storm syndrome (review in: Rubilar T et al., Mar Drugs 2021, 19, 267). Echinochrome is clinically used in cardiology and ophthalmology based on the unique properties to block various links of free radical reactions. Numerous studies have demonstrated the effectiveness of that compound in various disease models concerning ophthalmic, cardiovascular, cerebrovascular, inflammatory, metabolic, and malignant diseases (Kim HK et al., Marine Drugs 2021, 19/ 8, 412).
Due to the broad applicability of the underlying mechanism, the therapeutic potential of echinochrome A for various other candidate diseases such as inflammatory bowel disease, gastric ulcer, atopic dermatitis, and cystic fibrosis, has been vigorously investigated. It promotes the maintenance and regeneration of the intestinal epithelium, suggesting possible beneficial effects on the intestine when used as an oral medication (Ahn JS et al., Mar Drugs 2022, 20, 715). Given their biological properties, the extraction of polyhydroxynaphthoquinones for potential medical applications has received increasing attention (Roncoroni M et al., Mar Drugs 2024, 22(4), 163).
Rhinacanthin C is a naphthoquinone isolated from Rhinacanthus nasutus (an Acanthaceae plant native to tropical Asia and the western Indian Ocean) and has been shown to exhibit antiviral activity. It has a role as a metabolite, an antiviral agent and an antineoplastic agent. It is a carboxylic ester, an enol, an enoate ester and a hydroxy-1,4-naphthoquinone. It could have a future important place in the therapy of Parkinson disease since it has been shown to significantly restored the dopamine, norepinephrine and serotonin level in brain tissue of treated mice (Saleem U et al., J Food Biochem 2021, 45/4, e13677).
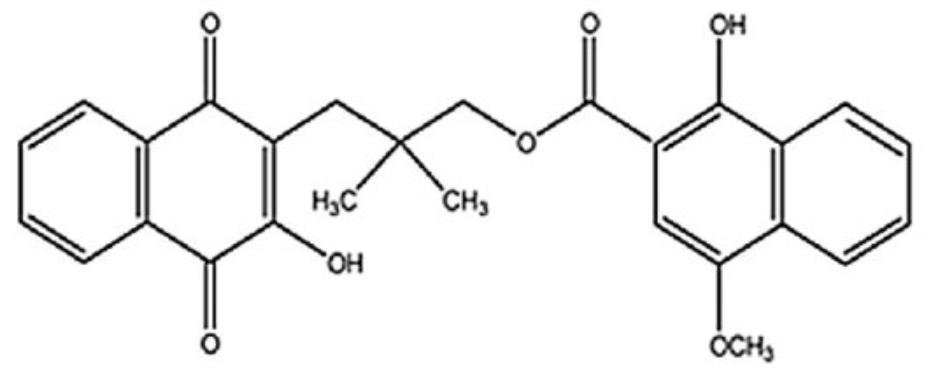 Rhinacanthin C
Rhinacanthin C
Binaphthyl compounds have been isolated from the fungus (Ascomycetes) Daldinia concentrica (Hashimoto T et al., Chem Pharm Bull 1994, 42, 1528). One of them is shown below.
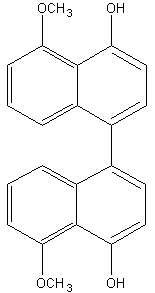
Phenanthrenes : several forms of phenanthrenes are present in in higher plants, mainly in Orchidaceae family but also in Dioscoreaceae, Combretaceae, Euphorbiaceae, Juncaceae and Hepaticae. The original phenanthrene molecule may be substituted in various positions by hydroxyl, methoxyl, methyl and/or prenyl groups. Most of them are monomeric but some dimeric and trimeric forms were described. As an example, the compound below (denthyrsinin) is reported in the orchid species Cymbidium pendulum, Dendrobium spp, Eulophia nuda, Nidema boothii, Scaphyglottis livida, Thunia alba. That compound, as others, displayed potent cytotoxic activities (review in Kovacs et al., Phytochemistry 2008, 69, 1084). Many plants producing phenanthrenes are used in traditional medicine, likely in connection with the cytotoxicity, anti-microbial, spasmolytic, anti-allergic, anti-inflammatory activities of the natural phenanthrenes present in these plants.
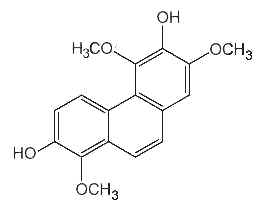
Denthyrsinin
New 9,10-dihydrophenanthrenes and phenanthrenes were isolated from Juncus setchuensis, a plant which has long been regarded as an antipyretic and detumescence agent in traditional Chinese medicine (Wang XY et al., J Nat Prod 2009, 72, 1209). Some of these compounds have shown strong antitumor and antialgal activities.
6,7-Dihydroxy- 2,4-dimethoxyphenanthrene has been shown to be the key bioactive compound from Chinese yam (Dioscorea opppsita) which has been widely used in invigorating intestines and improving long-term diarrhea according to the records of Chinese Pharmacopoeia (Li Q et al., Food Chem 4 October 2024, 141534). Furthermore, it has the strongest anti-inflammatory activity than other phenanthrenes.
Investigations have revealed that this compound was mainly digested and metabolized in the intestine, mainly into 2,6-dimethoxy-4-phenanthrenol and 1-methoxyphenanthrene which have the highest COX-2 enzyme inhibitory activity (Li Q et al., Food Chem 2025, 464, 141534).
 6,7-Dihydroxy- 2,4-dimethoxyphenanthrene
6,7-Dihydroxy- 2,4-dimethoxyphenanthrene
Hydroxylated derivatives of phenanthrene (dryhydroeffusol and effusol), isolated from Juncus effusus have anxiolytic and sedative effects, which could be used as novel anxiolytic natural products derived from herbal medicine (Singhuber J et al., Planta Med 2012, 78, 455).
Aristolochic acid 1 (8-methoxy-6-nitro-2H-phenanthro[3,4-d][1,3]dioxole-5-carboxylic acid) is a very potent and dangerous compound with a phenanthrene core. It is commonly found in the flowering plant family Aristolochiaceae and has carcinogenic, mutagenic, and nephrotoxic properties (Wikipedia).
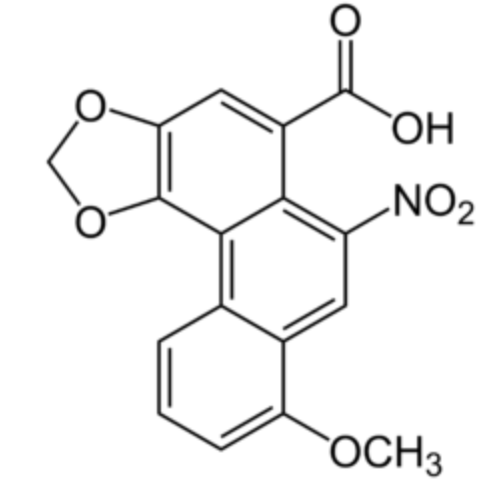
Aristolochic acid 1
Anthraquinones : the anthracene nucleus is present in several compounds detected in plants used in traditional medicine. Thus, anthraquinone is found in several plant species, mainly Aloes, fungi and lichens but also in insects where they play a role of pheromone. From the imperial courts of China to various tribes of the Americas, aloe has been revered as a healing plant with divine properties. Its symbolic significance has spanned continents, with names like “lily of the desert,” “silent healer,” and “elixir of long life” reflecting its cultural importance. Aloe vera latex contains about 80 different phenolic anthraquinones, some of which (aloe-emodin, aloin, chrysophanol, etc.) are major medicinal ingredients (Williams LD et al., Regul Toxicol Pharmacol 2010, 57, 90).
Studies on the phytochemical composition of Aloe vera (or barbadensis) and different species of rhubarb, Rheum genus, and Polygonum multiflorum (Polygonaceae), were undertaken as early as at the beginning of the 20th century, and provided information on the presence of a variety of anthraquinone (review in Kolodziejczyk-Czepas J et al., Phytochem Rev 2021, 20, 589). The chemistry and biological activities of anthraquinones and their analogues from marine-derived fungi has been reviewed (Ghoran SH et al., Marine Drugs 2022, 20(8), 474). The biological activities of hydroxyanthracene derivatives from Aloe species and their potential uses have been reviewed (Merino JJ et al., Phytochem Rev 2025, 24, 2387).
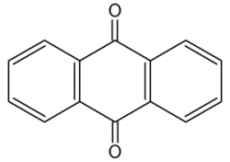
Anthraquinone
Aspergillus cristatus, a probiotic fungus isolated from Fuzhuan brick tea, produces various valuable anthraquinone metabolites metabolized which possess promising bioactivities and influence the tea fermentation process. Twelve anthraquinones were identified as (+)-variecolorquinone A, fallacinol, (+)1-O-demethylvariecolorquinone A, dermolutein, citreorosein, endocrocin, questin, rubrocristin, emodin, catenarin, physcion and erythroglaucin (Xie Z et al., Food Chem 30 January 2025, 143104). Functionally, these compounds demonstrated antioxidant, anti-inflammatory and antibacterial effects and hold promise as stable colorants and effective preservatives in food industry.
Hydroxyanthracene derivatives are a class of chemical substances naturally occurring in different botanical species and used in food to improve bowel function. The European Food Safety Authority (EFSA) has released advice concluding that hydroxyanthracene derivatives should be considered as genotoxic and carcinogenic unless there are specific data to the contrary. The hydroxyanthracene derivatives considered relevant for this risk assessment were those found in the root and rhizome of Rheum palmatum and/or Rheum officinale and/or their hybrids; leaves or fruits of Cassia senna and/or Cassia angustifolia; bark of Rhamnus frangula, bark of Rhamnus purshianus, in leaves of Aloe barbadensis and/or various Aloes pecies, mainly Aloe ferox and its hybrids.
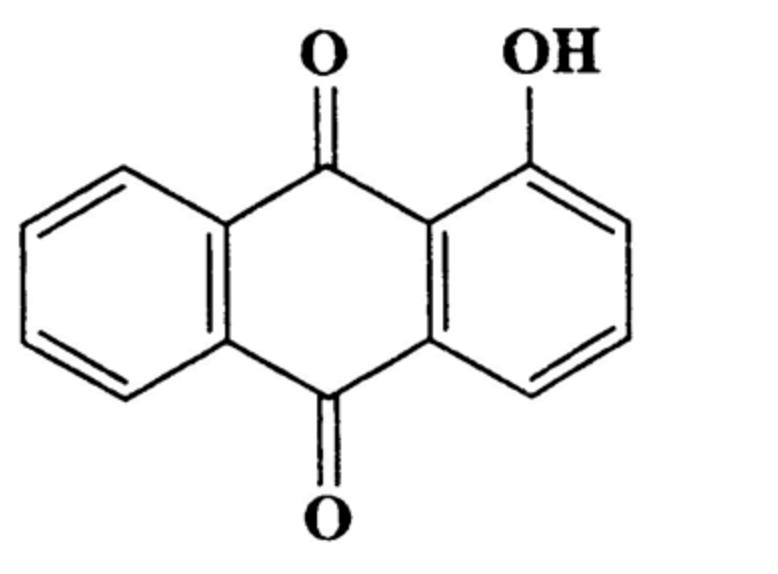 Hydroxy anthracene
Hydroxy anthracene
Among the various derivatives, emodin (6-methyl-1,3,8-trihydroxyanthraquinone) is the most important. It is an active component of several plants used in traditional Chinese medicine which have laxative, antibacterial and antiinflammatory effects. On the basis of information, the EFSA has concluded that hydroxyanthracene derivatives in food (i.e. Aloe extracts) can improve bowel function, but advised against long-term use and consumption at high doses due to potential safety concerns such as the danger of electrolyte imbalance, impaired function of the intestine and dependence on laxatives.
Chrysophanol is a member of the anthraquinone family. Pharmaceutical studies have shown that it exerts a number of biological effects, including anticancer and antimicrobial. The mechanism underlying the anti-inflammatory effects of chrysophanol is likely through the inhibition of caspase-1(Kim SJ et al., Molecules 2010, 15, 6436).
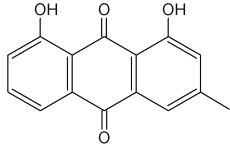
Chrysophanol
The anticancer potential of anthraquinones derived from marine sources and their genotoxic and mutagenic potential have been described in a review (Greco G et al., Mar Drugs 2021, 19, 272).
Several anthraquinones are considered as bioactive constituents in Cassiae seeds (Chunjuan Y et al., J Ethnopharmacol. 2015, 169, 305). The species the most studied, Cassia obtusifolia belongs to Leguminosae and known as ‘Jue Ming-Zi’ in China, which has been used in adjuvant therapy for various diseases such as hyperlipidemia, diabetes, Alzheimer’s disease, acute liver injury, inflammation, photo-phobia, headache, dizziness and hypertension.
One of these anthraquinones, obtusin, was shown to be a strong inhibitor of human thrombin, thus being a potential therapeutic strategy for the prevention and treatment of cardiovascular and thrombotic diseases.
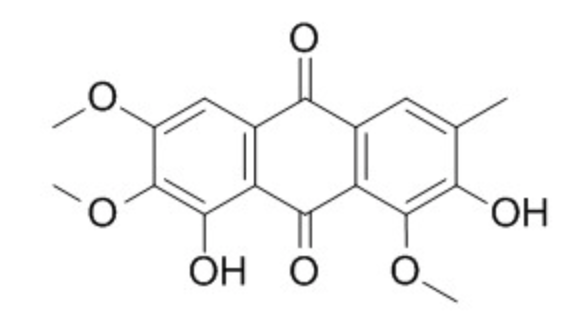
Obtusin from Cassiae seeds
Emodin (6-methyl-1,3,8-trihydroxyanthraquinone) can be isolated from rhubarb, buckthorn, and Japanese knotweed (Reynoutria japonica). It is specifically isolated from Rheum Palmatum. It has potent anti-inflammatory property as it inhibit lipoxygenase activities (Sharanya CS et al., Prostagl Other Lipid Mediators 2020, 150, 106453).
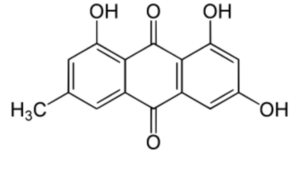
Emodin
Although not included in the living world but obviously derived by combustion of plants, many polycyclic aromatic hydrocarbons (PAHs), such as methylphenanthrene (3 cycles), triphenylene and chrysene (4 cycles), benzopyrene (5 cycles) and the coronene (6 cycles) have been identified in sediments dating from the early Triassic period to more recent times. Retene (1-methyl-7-isopropyl phenanthrene) derives here from the diagenesis of compounds which were abundantly produced by the early Palaeozoic bryophytes in the upper Silurian-lower Devonian period (Romero-Sarmiento MF et al., Org Geochem 2010, 41, 302).
Some have a structure of terpenes, they are discussed in another chapter. Many others are outside the scope of this work because they obviously come from contamination by petroleum products or their derivatives.
Several anthraquinones were isolated from Asteromyces cruciatus KMM4696 fungus associated with the brown alga Sargassum pallidum (Zhuravleva OI et al., Mar Drugs 2023, 21, 431). Several of them show a significant antimicrobial effects against Staphylococcus aureus growth. One of them, rubrumol, was among the most potent inhibitors.
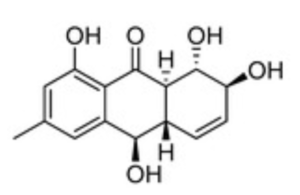 Rubrumol
Rubrumol
DISPERSIVE LIQUID-LIQUID MICROEXTRACTION
Lire la suiteDevenez membre et participez au développement de la Lipidomique au XXIème siècle.
S'inscrire