
UBIQUINONES
These molecules are also known as coenzyme Q or mitoquinones. They are involved in electron transport in mitochondrial preparations playing an essential role in the oxidation of succinate or NADH via the cytochrome system. They serves not only as a coenzyme but also, in their reduced forms, as antioxidants. They are synthesized de novo in all animal tissues and cannot thus be regarded as vitamins. Ubiquinones are present in all aerobic organisms, plants, animals (the name ubiquinone was proposed with reference to their ubiquitous occurrence) and bacteria, but are absent from Gram-positive eubacteria and the archaebacteria. They were discovered by the Morton’s group (Biochem J 1955, 59, 558) in animal fat but their quinonoid structure was revealed by Crane two years later (Biochim Biophys Acta 1957, 25, 220) in extracts from beef heart mitochondria. The compound had a 2,3-dimethoxy-5-methylbenzoquinone nucleus and a side chain of 10 isoprene units and was referred to as coenzyme Q 10 . Later, homologues with 6, 7, 8 and 9 units were isolated from other organisms, bacteria or higher organisms. The main form in man has 10 units but in rat has 9 units. Another system of nomenclature is used: ubiquinone(x) in which x designates the total number of carbon atoms in the side chain, it can be a multiple of 5. Thus, coenzyme Q10 is also named ubiquinone (50). Ubiquinone became the “official” name of the compound in 1975 (IUPAC-IUB commission).
See a review: “Biochemical, physiological and medical aspects of ubiquinone function” by Ernster L et al. (Biochim Biophys Acta 1995, 1271, 195).
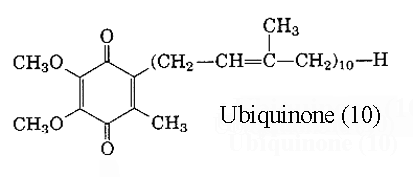
Ubiquinones accept one electron and are transformed into semiquinone radicals (UQH°) or two electrons to give ubiquinol (UQH2)
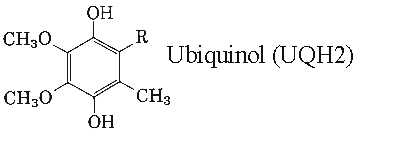
Studies have shown that ubiquinol exceeds the antioxidant activity of both vitamins E and C, supporting the maintenance of the body’s natural antioxidant levels.
Ubiquinol is essential for energy production in the mitochondria, its absence reducing skin cell renewal and activity. Ubiquinol has also been shown to boost collagen stimulation and elastane production to further support healthier skin (Schniertshauer D et al., Aging Res 2018:6354680).
Sudies have demonstrated that long-term supplementation of ubiquinol may help to maintain and improve cognitive performance and10 More recently, and that ubiquinol can help to reduce daily stress and improve sleep quality (Morikawa H et al., Jpn Pharmacol Ther 2019, 47, 1231). It has been shown that an oral supplementation of CoQ10 may increase clinical pregnancy rate when compared with placebo or no-treatment, in women with infertility undergoing assisted reproductive technology procedures, without an effect on live birth or miscarriage rates (Floroou P et al., J Assist Reprod Genet 2020, 37(10), 2377).
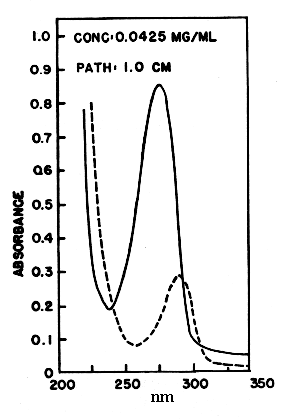
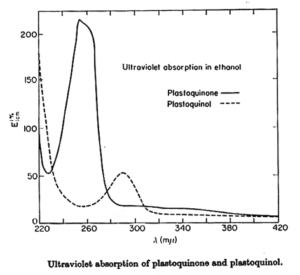
Coenzyme Q is reducible by sodium dithionite or borohydride to its hydroquinone form, and can in turn be reoxidized to the quinone by Ag2O or more slowly by oxygen. The absorption spectra of the two forms are shown below. The quinone form has a strong absorption band at 275 nm which disappears in the reduced form.
Ubiquinone-9: MW 794 Ubiquinone-10: MW 862
Ubiquinones are readily destroyed by heating in alkali , but in the presence of pyrogallol the destruction is minimized.
Idebenone is a synthetic analogue of coenzyme Q10 which has antioxidant properties.Iinitially developed by Takeda Pharmaceutical Company for the treatment of Alzheimer’s disease. it was investigated by the Swiss company Santhera Pharmaceuticals for the treatment of neuromuscular diseases. Several indications have been approved in some countries and many research are in progress in various nervous pathologies (treatment of neurodegenerative diseases) and skin disorders (Montenegro L et al., Nanomaterials 2018, 8(2). pii: E87).
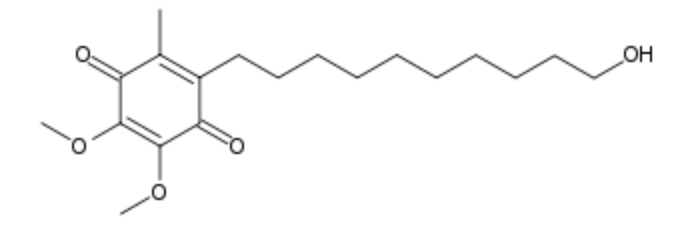
Idebenone
By disrupting the mitochondrial membrane potential and promoting mitophagy, Idebenone was shown to promote cell autophagy, thus suppressing the proliferation of triple-negative breast cancer cells (Zhang Y et al., Nutr Cancer 2024, 76, 379). Furthermore, Idebenone was shown to inhibit the development of these cells in vivo. Thus, Idebenone may be a promising therapeutic option for triple-negative breast cancer cells.
Idebenone was shown to possess also potential therapeutic application as a novel anti-inflammatory agent in systemic inflammatory diseases and sepsis (Choi Y et al., Antioxidants 2024, 13, 151).
A new quinone compound with estrogenic properties has been isolated from yam tuber (Dioscorea alata) (Cheng WY et al., J Agric Food Chem 2007, 55, 7350). This product was identified as hydro-Q9 chromene, a derivative of coenzyme Q9.
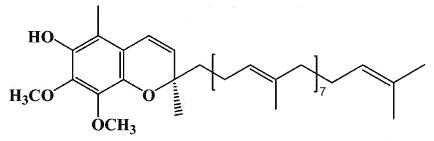
Hydro-Q9 chromene
The presence of that biologically active compound in yam extract likely provides basic evidence for a part of its beneficial effect for menopausal women.
A farnesylquinone has been isolated from the marine fungus Streptomyces nitrosporeus which was shown to have fat-reducing effects by enhancing mitochondrial β-oxidation rate and modifying energy metabolism genes transcription (Jia X et al., Mar Drugs 2019, 17, 336).
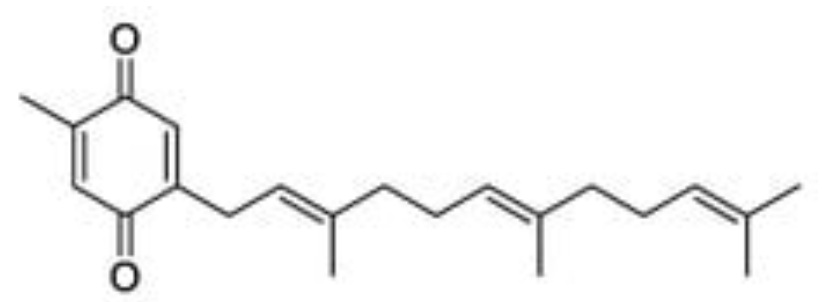 Farnesylquinone
Farnesylquinone
Mitoquinone mesylate is a synthetic analogue of coenzyme Q10 which has antioxidant effects. It has significantly improved bioavailability and improved mitochondrial penetration compared to coenzyme Q10 and has shown potential in a number of medical indications (Wikipedia).
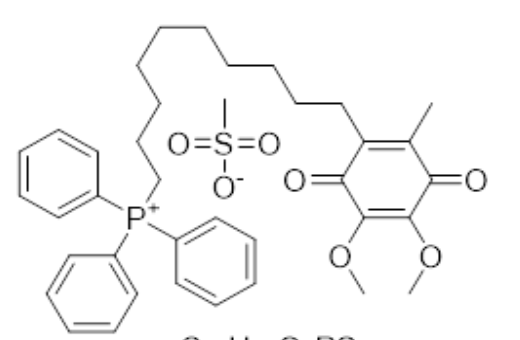 Mitoqinone mesylate
Mitoqinone mesylate
A substituted quinone was isolated in a lipid extract of lucerne by Kofler in 1946. The discovery of ubiquinone and of its role in mitochondrial electron transport stimulated studies of related substances. Crane FL (1959) suggested that this quinone was called plastoquinone because it appeared concentrated in the chloroplasts of higher plants. Rapidly, it appeared that plastoquinone plays a role analogous to that of ubiquinone in mitochondria but associated with the transformation of light into chemical energy. They are also present in algae and cyanobacteria.
The elucidation of the structure of plastoquinone was that of a 2,3-dimethyl-1,4-benzoquinone with a C45 side chain related to the isoprenoid alcohol, solanesol.
There are several plastoquinones with side chains of different length in position 5. They are designated as plastoquinone-n where n is the number of carbon atoms in the side chain or better as PQ-n where n indicates the number of isoprenoid units. n varies from 6 to 9.
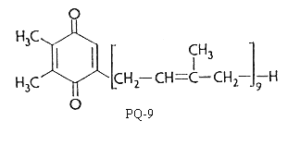
Plastoquinone is a yellow crystalline solid (PQ-9 has a MW of 748) soluble in most organic solvents. It has a characteristic UV spectrum with a maximum at 255 nm (molar abs. coeff.: 15700). On reduction (with sodium borohydride) a single maximum of lower intensity at 290 nm (molar abs. coeff.: 3440) is observed.
Several plastoquinone and plastohydroquinone analogues have been described in brown algae (Reddy P et al., Phytochemistry 2009, 70, 250). They differ primarily in the linear terpene chain moiety which has hydroxy and carbonyl groups in various positions. As an example, we give below the structures of the simplest (sargaquinone) and one of the most complex (fallaquinone) described in Sargassum fallax.
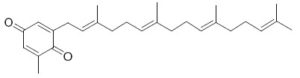
Sargaquinone
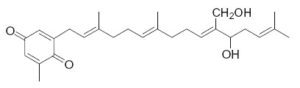
Fallaquinone
Many of these molecules displayed significant antioxidant, potent antiviral or moderate antitumour activities.
Among them, sargahydroquinoic acid is a major plastoquinone in Sargassum macrocarpum. It is a potent cyclooxygenase-2 Inhibitor and has shown the capacity to prevent inflammation and oxidative stress (Joung EJ et al., Inflammation 2021, 44, 2120). This report suggests that this plastoquinone might be a potential therapeutic agent in various inflammatory diseases.
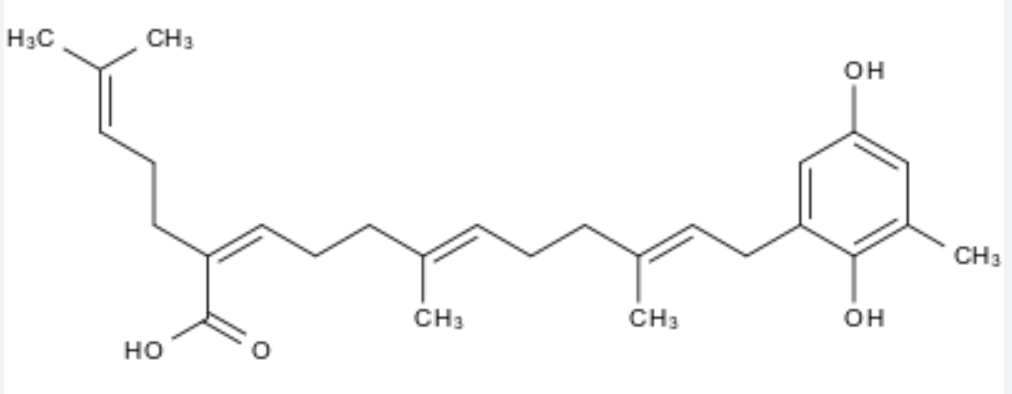 Sargahydroquinoic acid
Sargahydroquinoic acid
Various heterocyclic quinones have been described in sulphur-oxidizing archaebacteria, the benzothiophenoquinones.
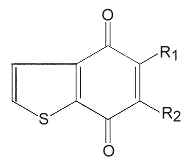
Several species of these quinones are present with menaquinones in the same bacteria. R1 may be -CH3 or -SCH3, and R2 is an isoprenoid chain, saturated or mono-unsaturated, with 3 to 6 isoprenoid units.
tert-Butylhydroquinone is a synthetic aromatic organic compound which is a derivative of hydroquinone, substituted with a tert-butyl group. In foods, It is used as an antioxidant to preserve unsaturated vegetable oils and many edible animal fats. It can be combined with other preservatives such as butylated hydroxyanisole. As a food additive, its E number is E319. While it has been determined as safe to consume at the concentration allowed in foods, a number of studies have shown that prolonged exposure to very high doses of butylhydroquinone may be carcinogenic (Wikipedia).
It was demonstrated that this compound can maintain better marketable fruit rate and suppressed activities of phospholipase D, lipase, and lipoxygenase (Yu Z et al., Food Chem 2024, 140041). Thus, it might play an important role in improving storability of postharvest fruit.
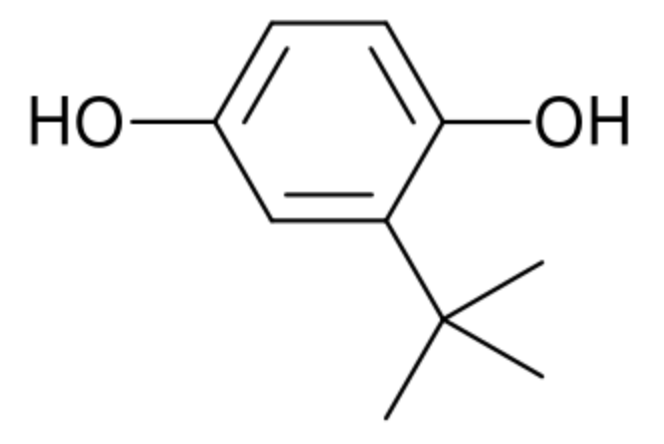
tert-Butylhydroquinone
Several alkylated hydroquinones have been described in vegetals.
As an example, we give below the common structure of cytotoxic compounds discovered in Tapirira guianensis, a tall tree from Mexico to Brazil, used as folk medicine (m = 8 and n = 5) (David JM et al., J Nat Prod 1998, 61, 287) and in Lannea welwitschii (lanneaquinol with m = 6 and n = 7) (Groweiss A et al., J Nat prod 1997, 60, 116).
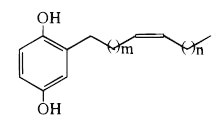
These two products displayed cytotoxic activity against human prostate cancer.
A large number of linear polyprenylhydroquinones (plastoquinones without methyl groups) have been isolated from sponges, especially from the Irciniidae family. Biological studies conducted on several of these molecules showed them to have a moderate antibacterial, antiviral, and anti-inflammatory activity. These linear polyprenylhydroquinones could be further divided in two main groups with polyprenylhydroquinones sulfates (De Rosa S et al., J Nat Prod 1995, 58, 1450) and hydroxylated polyprenylhydroquinones (Abed C et al., Mar Drugs 2011, 9, 1210).
OTHER BENZOQUINONES
These are groups of compounds containing two carbonyl groups on a saturated hexacyclic aromatic ring system (benzene ring), usually at ortho or para posi- tions (monocyclic). Quinones exhibit numerous biological activities such as neurological, antibacterial, antiplasmodial, antioxidantal, trypanocidal, antitumor, and anti-HIV, and these activities have been proven to be related to the redox properties of their carbonyl functions.
Naturally occurring quinones have been reported with a range of activity against insect, fungal, weed and other agricultural pests (Mozaina K et al., J. Agric. Food Chem 2008, 56, 4021). Quinones are widespread in the defensive secretion constituents of arthropods and the methylated quinones found in opilionids are of more limited occurrence (Eisner T et al., J. Chem. Ecol 1977, 3, 321). Methyl- and ethylquinones are well established components of the defensive secretions in many arthropods and their presence vary between species and even sexes (Gunbilig R et al., Scientific Reports 2009,, 3, 1865)
Thymoquinone (TQ; 2-isopropyl-5-methylbenzo-1, 4-quinone), the main active constituent of Nigella sativa and Monarda fistulosa, has been proven to have great therapeutic properties (Malik S et al., Drug Discov Today 2021, 26, 2716).
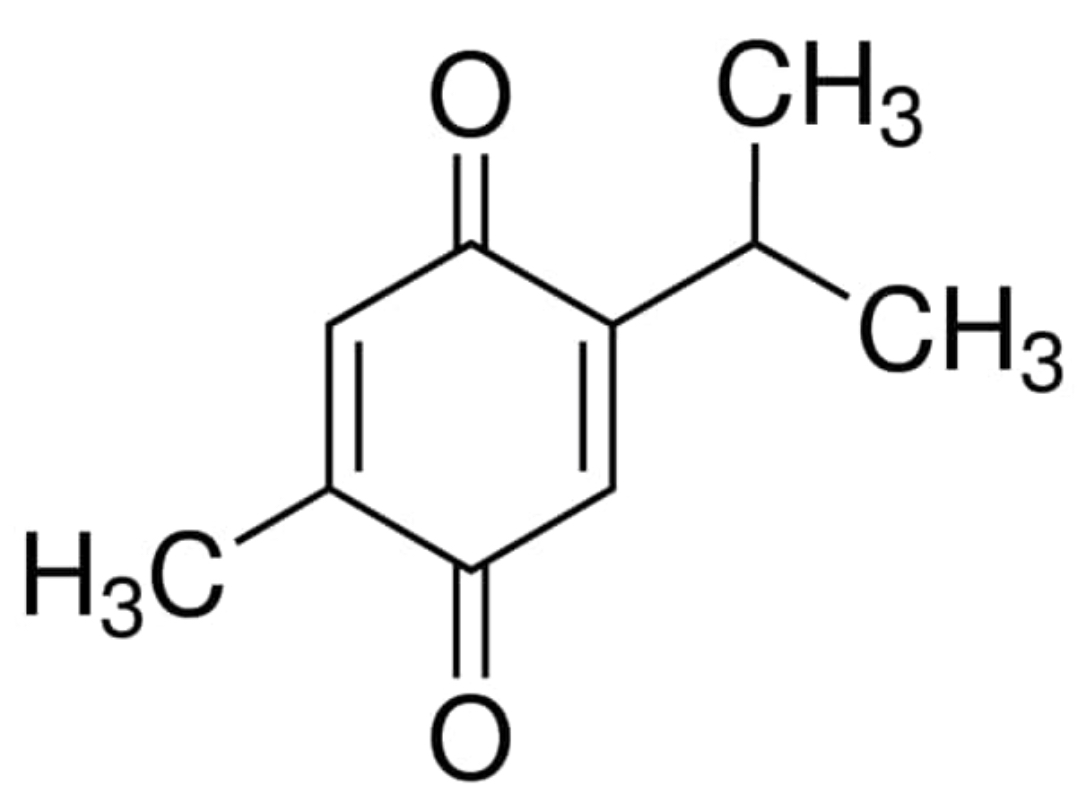 Thymoquinone
Thymoquinone
It was proposed as a promising natural compound with potential benefits for COVID-19 prevention and cure (Badary OA et al., Drug Des Devel Ther 2021, 15, 1819).
Precise observations have revealed that thymoquinone can impact signaling mechanisms that regulate proliferation and development in Dictyostelium discoideum (Alsaffar N et al., PLOS One 2023, 01 March).
Thymoquinone has also promising properties including anticancer and chemosensitizing peculiarities. Its anticancer power is accomplished by several aspects; including promotion of apoptosis, arrest of cell cycle and ROS generation (Mahmoud YK et al., Biomed Pharmacother 2019, 115, 108783). It has been demonstrated that UHRF1 poly-auto-ubiquitination induced by thymoquinone is involved in the DNA repair machinery recruitment (Almalki NAR et al., Int J Biochem Cell Biol 2024, 171, 106582). Further findings have highlighted the potential of combining traditional chemotherapies (5-fluorouracil) with thymoquinone and employed with nanobiotechnology, offering new insights into therapeutic strategies for colon cancer (Deng X et al., Food Biosci 12 June 2025, 107060).
Thymoquinone is also found in the black cumin (Nigella sativa) seeds. The black cumin seeds have long been used as a spice in the Mediterranean region and in Western Asian countries including India, Pakistan and Afghanistan. Investigations on the extracts of black cumin have indicated potent antitumor activities without serious toxic effects.
Devenez membre et participez au développement de la Lipidomique au XXIème siècle.
S'inscrire