
INTRODUCTION
Terpenoids (or isoprenoids), a subclass of the prenyl lipids (terpenes, prenylquinones, and sterols), represent the oldest group of small molecular products synthesized by plants and are probably the most widespread group of natural products.
Terpenoids can be described as modified terpenes, where methyl groups are moved or removed, or oxygen atoms added. Inversely, some authors use the term “terpenes” more broadly, to include the terpenoids.
During the 19th century, chemical works on turpentine led to name “terpene” the hydrocarbons with the general formula C10H16 found in that complex plant product. These terpenes are frequently found in plant essential oils, considered as the plant fragrance, . The term essential oil was created in the 16th century and refers to the theory of “Quinta essentia” proposed by Paracelsus (1493–1541). Paracelsus defined the role of alchemy by developing plant extracts and herbal medicines. He believed that the distillation process extracted the most significant part of the plant, namely, the “quintessence for healing” or “plant’s soul”, separating the “essential” constituents from the “nonessential”. Actually, an “essential oil” can be defined as a “product obtained from a natural raw material of plant origin, either by distillation with water or steam, or from the epicarp of fruits by a mechanical process, or by “dry distillation”. Essential oil is then separated from the aqueous phase by physical means” (review in: de Sousa DP et al. Biomolecules 2023, 13(7), 1144).
In general, essential oils are composed of approximately 20–60 components at different concentrations, but two or three components are usually present in large proportions (20–70%).
They are universally present in small amounts in living organisms, where they play numerous vital roles in plant physiology as well as important functions in all cellular membranes. The various functions of terpene natural products in the natural world have been reviewed (Gershenzon J et al., Nature Chem Biol 2007, 3, 408). On the other hand, they are also accumulated in many cases, and it is shown that the extraordinary variety they then display can be due to ecological factors playing an evolutionary role (Ourisson G, Pure Appl Chem 1990, 62, 1401). The Functions of representative terpenoids and their biosynthesis mechanisms in medicinal plants have been reviewed (Wang Q et al., Biomolecules 2023, 13(12), 1725). The antioxidant, anti-inflammatory, antitumor, and antimicrobial activities of monoterpenes and sesquiterpenes have been rewied (de Sousa DP et al. Biomolecules 2023, 13(7), 1144).
More than 50,000 terpenoids have been isolated from both terrestrial and marine plants, and fungi. In contrast, only some compounds have been identified in prokaryotes. Currently, about 3000 aromatic plants are known to produce essential oils as an important part of their secondary metabolism, 300 of which are commercially significant due to their medicinal and industrial values.
The first study of bacterial terpenes grew out of an investigation of the characteristic odor of freshly plowed soil reported in 1891 by the famous French chemist M. Berthelot (Berthelot M, et al., C R Acad Sci 1891, 112, 598). They noted that a volatile substance apparently responsible for the typical earthy odor of soil could be extracted from soil by steam distillation but they could not assign a structure to the odor constituent.
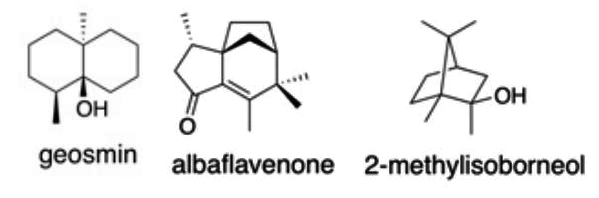
Some 75 years later M.N. Gerber studied the characteristic odor of cultures of Actinomycetales microorganisms, which are widely distributed in soil, and determined the structure of a C12 degraded sesquiterpene alcohol, the geosmin (means earth odor), likely detected by Berthelot (Gerber NN, et al., Appl Microbiol 1965, 13, 935). Later, numerous volatile terpenes have been detected in streptomycetes. The three most commonly detected are geosmin, 2-methylisoborneol and the tricyclic α,β-unsaturated ketone albaflavenone. The two terpene alcohols are the most frequently found secondary metabolites in some actinomycetes, filamentous blue-green algae (Cyanobacteria), and Myxobacteria, and also in a small number of fungi. Enzymatic studies have suggested that genes encoding terpene synthases are widely distributed in bacteria and that these genes represent a fertile source for discovery of new natural products (Yamada Y et al., PNAS 2015, 112, 857).
Geosmin, along with 2-methylisoborneol, account for the majority of biologically-caused taste and odor outbreaks in drinking water worldwide. Geosmin has a distinct earthy or musty odor. It is also responsible for the earthy taste of beetroots and a contributor to the strong scent (petrichor) that occurs in the air when rain falls after a dry weather.
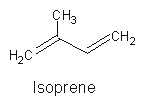
All terpenoids may be defined as a group of molecules whose structure is based on a various but definite number of isoprene units (methylbuta-1,3-diene, named hemiterpene, with 5 carbon atoms).
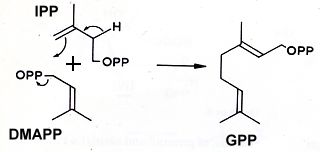
Terpenoids are extraordinarily diverse but they all originate through the condensation of the universal phosphorylated derivative of hemiterpene, isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP) giving geranyl pyrophosphate (GPP).
In higher plants, IPP is derived from the classic mevalonic acid pathway in the cytosol but from the methylerythritol phosphate pathway in plastids. It is generally accepted that the cytosolic pool of IPP serves as a precursor of sesquiterpenes, triterpenes, sterols and polyterpenes whereas the plastid pool of IPP provides the precursors of mono-, di- and tetraterpenes (Bohlmann J et al., Proc Natl Acad Sci USA 1998, 95, 4126). Some exceptions have been described showing that interactions between the two biosynthetic pathways may exist (Dudareva N et al., Proc Natl Acad Sci USA 2005, 102, 933).
A metabolic map giving the products of isoprene metabolism and their pathways may be found at the Prof Nicholson web site
A rational classification of the terpenes has been established based upon the number of isoprene (or isopentane) units incorporated in the basic molecular skeleton:
| Terpenes | Isoprene units |
Carbon atoms |
|
| 1 | Monoterpenes | 2 | 10 |
| 2 | Sesquiterpenes | 3 | 15 |
| 3 | Diterpenes | 4 | 20 |
| 4 | Sesterterpenes | 5 | 25 |
| 5 | Triterpenes | 6 | 30 |
| 6 | Carotenoids | 8 | 40 |
| 7 | Rubber | > 100 | > 500 |
Mono-, sesqui-, di-, and sesterterpenes contain the isoprene units linked in a head to tail fashion. The triterpenes and carotenoids (tetraterpenes) contain two C15 and C20 units respectively linked head to head.
Several terpenes are hydrocarbons, but oxygen-containing compounds such as alcohols, aldehydes or ketones are also found. These derivatives are frequently named terpenoids.
Mono- and sesquiterpenes are the chief constituents of the essential oils while the other terpenes are constituents of balsams, resins, waxes, and rubber.
Oleoresin is a roughly equal mixture of turpentine (85% C10-monoterpenes and 15% C15- sesquiterpenes) and rosin (C20-diterpenes) that acts in many conifer species to seal wounds and is toxic to both invading insects and their pathogenic fungi (Steele CL et al., Plant Physiol 1998, 116, 1497). Turpentine fraction contains a range of 20 insect and microbial toxins, such as limonene and carene, and other biologically-active agents that often act synergistically to discourage insect predation. Turpentine also acts as the solvent able to transport the higher molecular weight diterpenoid resin acids (rosin fraction) to the site of injury. Upon exposure to the atmosphere, the volatile turpentine evaporates, leaving a resin that oxidatively polymerizes to form a barrier that seals the wound, often trapping insect invaders and microbial pathogens (review in Phillips MA et al., Trends Plant Sci Rev 1999, 4, 184).
A number of inducible terpenoid defensive compounds (phytoalexins) from angiosperm species are well known (Stoessl et al., Phytochemistry 1976, 15, 855). These include both sesquiterpenoid and diterpenoid types.
Isoprenoid units are also found within the framework of other natural molecules. Thus, indole alkaloids, several quinones (vitamin K), alcohols (vitamin E, vitamin A formed from β-carotene), phenols, isoprenoid alcohols (also known as terpenols or polyprenols) also contain terpenoid fragments. The origin of the ubiquitous isoprene unit and its conversion into various compound has been extensively studied.The biogenesis, molecular regulation and function of plant terpenoids has been extensively reviewed by Bouvier F et al. (Prog Lipid Res 2005, 44, 357).
According to Bohlmann J et al. (PNAS 1998, 95, 4126), there are in excess of 1000 monoterpenes, more than 7000 sesquiterpenes and more than 3000 diterpenes.
HISTORY
Terpenes history spans various civilizations. As they are largely found in essential oils, they were used in the Ancient Egypt for various religions aims. Camphor was introduced in Europe from the East by the Arabs around the 11th century.
The process of obtaining plant essential oils by fatty extraction was known by the early Middle Ages. In the 12th century, Arnaud de Villanosa described distillation of oils from rosemary and sage. He made an “oleum mirabile” from oils of turpentine and rosemary. It is noticeable that some 60 oils were described in the Nuremberg edition of “Dispensatorium valerii cordi” written in 1592.
Analyses of oils of turpentine were made in 1818 by JJ Houston de la Billardière. Dumas proposed in 1866 the name “terpene”, derived from turpentine, instead of camphor for crystalline oxygenated substances extracted from essential oils. In 1887, Wallach O proposed that one isoprenic unit of 5 carbon atoms (C5H8) is always present in the molecule of terpenes (Justus Lieb Ann Chem 1887, 238, 78).
The structure of camphor was established by Bredt in 1893, that of pinene by Wagner in 1894 and that of citral by Tiemann in 1895. b-Carotene was isolated in 1837 by Wackenrodder from carrots, and its correct molecular form was determined in 1907 by Willstätter.
The period since 1945 has seen an extensive explosion in natural product chemistry due to the advent of chromatographic and spectroscopic techniques. The discovery of the isoprene unit is the basis of the concept of the “isoprenic rule” edicted in 1953 by Ruzicka L (Experientia, 1953, 9, 357) and completed by Lynen F et al. (Angew Chem 1958, 70, 738) and Bloch K et al. (J Biol Chem 1959, 234, 2595).
Mevalonic acid was shown in 1956 to be a biosynthetic precursor of cholesterol (Tavormina PA et al., J Am Chem Soc 1956, 78, 4498) and later, its incorporation into a number of terpenoids has been demonstrated. Actually, an increasing number of terpenoids are described in the plant kingdom and many of them were shown to have important biological activities. Thus, several sesquiterpenes and diterpenes have antibiotic properties, some sesquiterpenes and diterpenes are insect and plant hormones, respectively.
Devenez membre et participez au développement de la Lipidomique au XXIème siècle.
S'inscrire