
These lipids (known also as monoglycerides) are fatty acid monoesters of glycerol and thus, due to the orientation of that molecule, two isomeric forms exist:
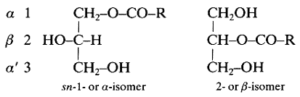
R is a saturated or an unsaturated hydrocarbon chain. The external carbons are frequently named a and the central one b.
Some monoglycerides are monoesters of terpenic or phenolic acids, in animals or vegetals, respectively.
Monoacylglycerols are found in very low amounts in cell extracts but are intermediates in the degradation of triacylglycerols or diacylglycerols (lipolysis). When diacylglycerols are hydrolyzed by lingual and pancreatic lipases, sn-2-monoacylglycerols are formed but an important proportion is isomerized in the duodenum in sn-1 and sn-3-monoacylglycerols which again can be hydrolyzed (the sn-2 position is lipase resistant). This physiological mechanism is based on the acyl migration from the sn-2 position to the sn-1 or 3 position, migration which is also commonly observed experimentally during purification and in acidic medium. The use of borate ions, added in solvents or on silica gel plates, tends to prevent such artifact.
The abiotic synthesis of monoacylglycerols has been shown to be possible in the laboratory under simulated hydrothermal conditions (Rushdi AI et al., Orig Life Evol Biosph 2006, 36, 93). These results indicate that condensation reactions under these primitive physical conditions may be the source of compounds for abiotic membranes which are candidates for micelle/vesicle formation at the beginning of life.
Extensive efforts have been made for high yield of monoacylglycerols from various reaction systems, using either chemical or enzyme as catalysts (Zhong N et al., Eur J Lipid Sci Technol 2014, 116, 97).
Some monoacylglycerols may have precise biological properties. Thus, it was demonstrated that a specific monoacylglycerol molecular species, 2-arachidonoylglycerol, is an endogenous ligand for cannabinoid receptors (CB1 and CB2) in brain tissue (Sugiura T et al., Biochem Biophys Res Comm 1995, 215, 89) and in gut (Mechoulam R et al., Biochem Pharmacol 1995, 50, 83). They are named endocannabinoids.
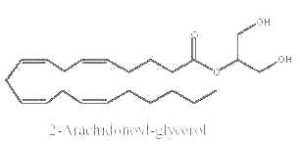
2-arachidonoylglycerol
Previously believed to be simply an intermediate in tri- and diglyceride metabolism or an alternative source of arachidonic acid, 2-arachidonoylglycerol has lately attracted renewed interest from lipid biochemists and pharmacologists. This is due to the finding of its cannabimimetic activity. The amount of this species was recently shown to be about 5 nmol/g tissue, this being about half of the total monoacylglycerols in the nervous tissue (Kondo S et al., FEBS Letters 1998, 429, 152). It was determined that 2-arachidonoylglycerol produced under ethanol action by hepatic stellate cells mediates, via the cannabinoid receptors of hepatocytes, an ethanol-induced steatosis through increasing lipogenesis and decreasing fatty acid oxidation (Jeong W et al., Cell Metab 2008, 7, 227). It has been suggested that 2-arachidonoylglycerol hydrolysis in the brain generates prostaglandins which promote neuroinflammation (Nomura DK et al., Science 2011, 334, 809). A review of the wide array of (patho)physiological functions, such as emotion, cognition, energy balance, pain sensation and neuroinflammation may be consulted (Baggelaar MP et al., Prog Lipid Res 2018, 71, 1-17).
A complete review on this lipid may be found in the web site of Biochimica (Moscow) and in review papers (Di Marzo V et al., Lipids 1999, 34 supl, S319; (Howlett AC, Prost Lipid Med 2002, 68-69, 619). Its metabolism has been reviewed by Bisogno T et al. (Bisogno T et al., Pharmacol Biochem Behav 2005, 81, 224). Briefly, the most likely biosynthetic pathway of 2-arachidonoylglycerol involves the hydrolysis of phosphatidylinositol (4,5)-bisphosphate by phospholipase C which generates 1,2-diacylglycerol, which is hydrolyzed to 2-arachidonoylglycerol by diacylglycerol lipase. An overview of the biochemistry and pharmacology of 2-arachidonoylglycerol has been released (Hansen HS et al., Eur J Lipid Sci Technol 2006, 108, 877).
Because of its low abundance in living tissues, quantification of 2-arachidonoylglycerol presents numerous technical challenges. A purification using a C18 solid-phase column and a GC/MS analysis permit precise and reproducible quantification in tissue samples (Hardison S et al., Prost Lipid Mediat 2006, 81, 106).
Other fatty acid substitutes, 2-palmitoylglycerol, 2-linoleoylglycerol, 1(3)-palmitoylglycerol and 1(3)-stearoylglycerol fail to bind to CB1 or CB2 receptors with reasonable affinity or mimic 2-arachidonoylglycerol’s biological effects. A quantitative method for the profiling of these various endocannabinoids present in rat brain by mass spectrometry has been described (Williams J et al., Anal Chem 2007, 79, 5582).
Among the various acyl glycerol analogues, an ether-linked analog of 2-arachidonoylglycerol (2-arachidonoyl glyceryl ether or noladin) was described (Hanus L et al., PNAS 2001, 98, 3662). Noladin was identified in relatively high amounts in dissected thalamus.
It has been reported on the occurrence and bioactivity of monoacylglycerols prepared from the marine diatom Skeletonema marinoi, with selective cytotoxic activity against the haematological cancer cell line U-937 and the colon cancer cell line HCT-116, acting as pro-apoptotic agents (Miceli, M et al., Mar Drugs 2019, 17, 625). The mechanism of action was based on involves the induction of apoptosis through caspase 3/7 activation.
Another monoacylglycerol species (2-sciadonoylglycerol) with cannabinomimetic activity has been found in seeds of a higher plant (Umbrella pine, Sciadopitys verticillata). This molecule contains a rare unsaturated fatty acid, 5,11,14-eicosatrienoic acid (or sciadonic acid) (Nakane S et al., Biol Pharm Bull 2000, 23, 758). Its role in the vegetal has not been determined. Another species, 1-butyrylglycerol, is produced during adipocyte differentiation and seems to be a key regulatory molecule in angiogenesis (Dobson DE et al. Cell 1990, 61, 223).
Monoacylglycerols containing epoxyeicosatrienoic acid at the 2 position of glycerol were identified as compounds having also a high affinity for cannabinoid receptors (Chen JK et al., J Biol Chem 2008, 283, 24514). These results suggest a functional link between the cytochrome P450 enzyme system generating epoxy fatty acids and the endocannabinoid system.
A series of specific acylglycerols have been isolated from the nonpolar fraction of propolis resin, having bioactive properties. These compounds are a series of monoglycerides containing 3,8-dihydroxy fatty acids, many of which are further acylated with acetic acid residues. The dihydroxy fatty acids are C18 to C24, the most abundant being C20 and C22. These acylglycerols were found to have strong antiproliferative activity against three human gastrointestinal cell lines (Bloor S et al., J. Nat. Prod. 2019, 82, 2359).
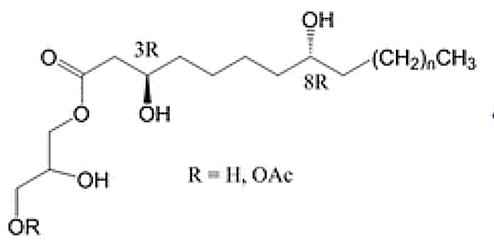 Propolis acylglycerols
Propolis acylglycerols
Monoacylglycerol were shown to be important in the constitution of cutin polymer (Graca J et al., Phytochemistry 2002, 61, 205). Cutin is the structural component of the plant cuticle, the outermost layer of aerial organs of higher plants. Waxes embedded in the cutin make the cuticle an efficient barrier against desiccation and gas exchange and pathogen attack. Cutin polyester is typically composed of esterified hydroxy-fatty acids with 16, 18 and 22 carbon-chains and one terminal hydroxyl (w-position) and other hydroxyl groups in secondary positions. The cutin polymer has been found to be based on the inter-esterification of hydroxyacids (head-to-tail in a linear form or cross-linked) and of glycerol esterified with various hydroxy-fatty acids.
Similar structures were described, suberin, which is a lipidic polyester present in tree barks, tuber skins and abscisic tissue of falling leaves. It is also formed in plant after wounding. Upon depolymerization, cork suberin releases a mixture of monomers and oligomers, including monoacylglycerols of monoacid (C22), of ω-hydroxyacids (C16, 18:1, 22, 24), and of a,w-diacids (C16, 18, 18:1, 22) (Graça J et al., Chem Phys Lipids 2006, 144, 96). Glycerol is a major compound of this polyester, constituting up to 20% by weight of suberin in oak, cotton and potato (Graça J et al., Agric Food Chem 2000, 48, 5476). The current model describes
A monoglyceride containing 26-hydroxy-26:0 fatty acid has been isolated from the root bark of Pentaclethra eetveldeana, used in Zairian traditional medicine for the treatment of hemorrhoids, malaria and epilepsy (Byla B et al., Phytochemistry, 1996, 42, 501).
Monoacylglycerols are the most polar components of simple lipids (they have only one hydrocarbon chain and 2 alcohol groups) and, thus, need care to prevent their loss in hydrophilic solutions and on chromatographic columns. Furthermore, they have detergent properties, hence they easily form micelles in water solutions.
Monoacylglycerols with saturated or unsaturated fatty acids are by far the most commonly used food surfactants. Surfactants are used in the food industry to prepare food products and increase their shelf life. They give to emulsions their stability and the required viscosity. The first use of monoacylglycerols on an industrial scale was, more than 50 years ago, for making margarine where they emulsify the water phase in oil and fat phase. They are now currently included in low-calorie spreads, peanut butter, ice cream to control their texture, starch-base food (macaroni, noodles, potato products…) and baking industry.
Monoacylglycerols are used also as raw materials for making more lipophilic or more hydrophilic molecules utilized in cosmetics and food industry. Esters are made with acetic, lactic, succinic and citric acids to emulsify commercial products.
These food additives are permitted for use in foods after they have been tested in toxicological studies and are assigned an E number by a EEC Council Directive or a GRAS number after regulation by the US Food and Drug Administration. Thus, monoacylglycerols, their acetic, lactic and citric esters are labeled as E471, E472a, E472b, and E472c, respectively. In the US, only monoacylglycerols are generally recognized as safe. No established acceptable daily intakes are defined for these surfactants.
Acetic acid esters of monoglyceride, called acetylated monoglyceride, can improve the quality of fats, for example, margarine and are available as a solvent, lubricant, plasticizer for vinylacetate, etc. Although they have no function as emulsifiers, they are usable for foaming fats and oils by themselves or in combination with other emulsifiers.
Lactic acid esters of monoglyceride, called lactylated monoglyceride, are used in shortening for cakes, desserts and foaming for cream by themselves or in combination with monoglycerides.
Succinic Acid esters of monoglyceride, called succinylated monoglyceride, form a complex with starch which is able to react with protein. They are used as dough modifying agents and emulsifiers for shortening.
Citric acid esters of monoglyceride, called citrated monoglyceride, are highly hydrophilic emulsifiers and are used for margarine, dairy products such as, coffee whitener and cream. They are also used as emulsion stabilizer for mayonnaise and dressing.
Since a long time it was shown that a fatty acid esterified to glycerol has stronger antimicrobial activity than the corresponding free acid (Kabara J et al., in: Davidson P et al., Eds, Antimicrobials in Food, CRC Press, Boca Raton, USA 2005, pp. 327–360). The structure-function relationship for saturated and unsaturated esters follows the activity of their respective fatty acids (Kabara JJ, JAOCS 1984, 61, 397). Monolaurin is the most frequently used antimicrobial supplement in food manufacture. It was first reported as an antifungal in margarine. Monocaprin has also been proposed for that use. Monocaprylin has been proposed as effective agent for antimicrobial textiles and lining for footwear, including those designed for diabetics (Vltavska P et al., Eur J Lipid Sci Technol 2012, 114, 849). Monolaurin is the most potent antibacterial agent against several species of Bacillus and Staphylococcus. Its is commonly used in foods and cosmetics.
Sulfated derivatives (on sn-3 position) of monoglycerides from coconut oil (cocomonoglyceride sulfate – CMGS) have been known for a long time in cosmetic industry as soon as 1935 by the Colgate-Palmolive Co to obtain an efficient shampoo formulation. To day CMGS are obtained directly from coconut oil in a solvent-free two-stage process. In the first stage coco-monoglycerides are obtained by simple transesterification of coconut oil with glycerol. This raw material is converted to CMGS by reaction with sulfur trioxide gas. The product is then neutralized with aqueous sodium hydroxide. Because of its technical application properties CMGS is predestined for use in cosmetic products such as shower gels and foam baths or shampoos. In these products, they are used in combinations of alkyl polyglycosides (Behler A et al. Fett/Lipid 1998, 98, 309).
Several terpenoic monoacylglycerols have been isolated from the mollusk nudibranch Archidoris odhneri (Andersen RJ et al., Tetrahedron Lett 1980, 21, 797). The acylated group was a sesquiterpene, farnesic acid.
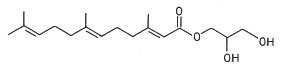
Farnesic acid monoglyceride
In another nudibranch, Archidoris montereyensis, the acylated group is a diterpenoic acid (Gustafson K et al., Tetrahedron Lett 1984, 25, 11).
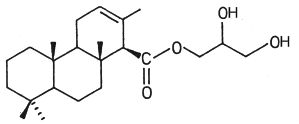
Diterpenoic acid monoglyceride
In the same mollusk species and in the nudibranch Sclerodoris tanya, several monoglycerides with a sesquiterpenoic acid was isolated. All these compounds may be a part of the cutaneous chemical used as antifeedants by these animals (Krug PJ et al., Tetrahedron 1995, 51, 11063).
In vegetals, phenolic monoacylglycerols have been isolated. Thus, p-coumaroyl monoglycerides have been described in the medullae of Juncus effusus (Dong-Zhe J et al., Phytochemistry 1996, 41, 545). This plant material is used as a traditional medicine in the Chinese pharmacopoeia. The coumaroyl group was determined to be acylated in sn-1 or in sn-2 position.
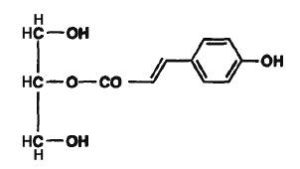
2-O-p-Coumaroyl monoglyceride
![]()
It is well known that many marine invertebrates, including sponges, corals, mollusks, starfish, holothurians, crabs, and ascidians as well as some marine algae are well-established sources of a variety of natural 1-O-alkyl-sn-glycerol ethers ; for a review, see : Chem Phys Lipids 2011, 164, 315 – Urate K et al., JAOCS 1996, 73, 819).
The major monoalkyl ethers (or alkoxyglycerols) are: 1-O-hexadecylglycerol (16:0 alkyl or chimyl alcohol), 1-O-octadecylglycerol (18:0 alkyl or batyl alcohol) and 1-O-octadec-9-enyl glycerol (18:1 alk-9-enyl or selachyl alcohol). The trivial names are based on the fish species from which they have originally isolated.
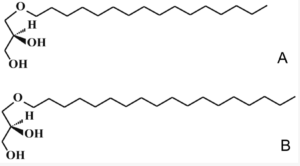
A—chimyl alcohol, B—batyl alcohol.
These components are found free in small quantities in various marine oils but are most frequently found either as alkyl-diacylglycerols (similar to triacylglycerols) in elasmobranch fish or as constituents of particular phospholipids in all kinds of cell membranes. In a few liver oils of elasmobranch these ether lipids form a relatively large proportion of the total unsaponifiable matter (about 10 per cent in Squalus acanthias, 37 per cent in Chimoera monstrosa and 50-80 per cent in Centrophorus spor Scymnorhinus sp).
Studies of the Holothurian class of sea cucumber, including Cucumaria japonica, C. okhotensis, C. fraudatrix and Stichopus japonicas, have identified several ether lipids, specifically 1-O-alkylglyceryl ethers (Isay SV et al., Comp. Biochem. Physiol. 1976, 55B, 301 – Rybin V et al., Nat. Prod. Commun. 2007, 2). Monoalkylglyceryl ethers were also detected in high amounts in Cucumaria frondoss viscera (Abuzaytoun R et al., Mar Drugs 2022, 20(7), 435).
The first evidence of the existence of these lipids seems to have been reported by Dorée C (1909) in England and Kossel A et al. (1915) in Germany. They isolated an unsaponifiable fraction of lipids from starfish they named “astrol”, which appeared later to be batyl alcohol and the occurrence of three similar alcohols was demonstrated in 1922 (Tsujimoto M et al., Chem Umschau 1922, 29, 27; 1924, 31, 13). The complete proof of their chemical structure was provided in England by Davies WH et al (1933). Later, it was demonstrated that these compounds are present in the form of fatty acid esters, each of the free hydroxyl groups being combined with a higher fatty acid (André E et al., Compt Rend 1932, 195, 627). Monoalkylethers are also present in human and cow milk, and in haematopoietic organs such as bone marrow and spleen. Several free monoalkylethers have been isolated from the jelyfish Nemopilema nomurai (Liu J et al., Nat Prod Sci 2009, 15, 71). The carbon chain with 12, 14, 16 or 18 carbon atoms is either saturated or has only one double bond. These compounds had no suppressive effect on the pro-inflammatory mediators in murine macrophage cells. 1-Glyceryl ethers have been isolated from the red alga-sponge association Ceratodictyon/Haliclona (Akiyama T et al., J Nat Prod 2009, 72, 1552). These glyceryl ethers species (ceratodictyols) have a 16-carbon chain with a ketone at C5 or C7 and a double bond in various locations. They exhibited weak cytotoxic activity against HeLa human cervical cancer cells.
Natural alkylethers have several biological activities such as stimulation of immunological (Palmblad J et al., Scand J Clin Lab Invest 1990, 50, 363) and haematopoietic functions. They also showed significant anti-inflammatory activity when given orally in rats (Burford RG et al., Arch Int Pharmacodyn Ther 1968, 173, 56).
For a long time it is well known that the oral administration of monoalkylethers reduces the negative effects of radiations received in radiation therapies (Brohult A et al., Acta Chem Scand 1970, 24, 730). Some monoalkylethers can suppress the growth of solid tumor when administered through various routes (Ando K et al., Cancer Res 1972,32, 125). It was also shown that these activities are in connection with an inhibition of cell signaling pathways (Arthur G et al., Biochim Biophys Acta 1998, 1390, 85). These anti-metastasis activities may be related to anti-angiogenic properties (Pedrono F et al., Cancer Lett 2007, 351, 317; Pedrono F et al., Nutr Cancer 2004, 48, 64). Alkylglycerols with 16:1 and 18:1 are the most potent compounds on tumor growth and lung macrometastasis number, while other compounds (with 16:0 and 12:0) have weaker activities. A review of the multiple beneficial effects of alkylglycerols from shark liver oil may be found in a documented article (Deniau AL et al., Mar Drugs 2010, 8, 2175) and an update on their therapeutic role in another article (Iannitti T et al., Mar Drugs 2010, 8, 2267).
A review has summarized the hematopoietic and immunostimulating properties of alkyl glycerol ethers and their role in the nervous system and aging of the body are also briefly described (Sultanov R et al., Mar Drugs 2023, 21(1), 4). The authors conclude that these lipids reduce many of the negative effects of stress. Therefore, they consider it promising to study alkyl glycerol ethers in order to develop practical recommendations for their use in stressful situations, adaptation to extreme environmental conditions, and also in the treatment of gastrointestinal ulcers.
Among the various acyl glycerol analogues, 2-arachidonoyl glyceryl ether, a monoalkyl ether analog of 2-arachidonoylglycerol was first used as a tool to explore in vivo the biological activities of 2-arachidonoylglycerol (Sugiura T et al., J Biol Chem 1999, 274, 2794). This ether lipid, which is resistant to lipases, was later reported to be present in pig brain and to be a specific CB1 agonist. It was named noladin ether (Hanus L et al., PNAS 2001, 98, 3662).
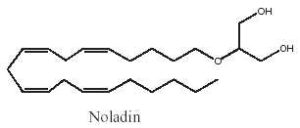
Noladin ether has much greater selective for CB1 over CB2 receptors; however, its activity as a CB1 agonist may be less than that exhibited by 2-arachidonoylglycerol.
Devenez membre et participez au développement de la Lipidomique au XXIème siècle.
S'inscrire