
Steroids form an important group of compounds based on the fundamental saturated tetracyclic hydrocarbon : 1,2-cyclopentanoperhydrophenanthrene (sterane or gonane).
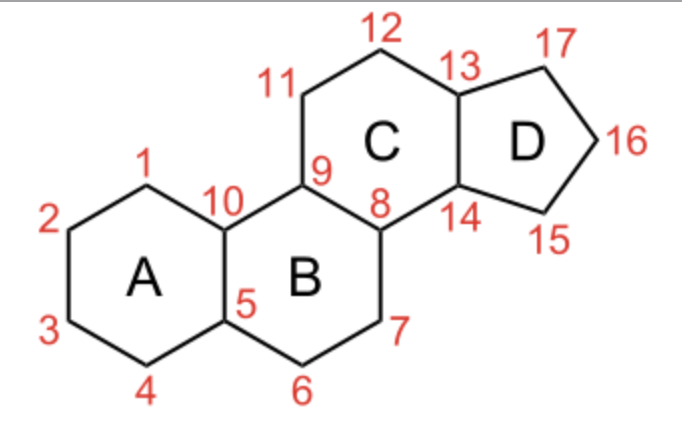 Sterane
Sterane
This nucleus, partially or completely hydrogenated, is generally substituted by methyl groups at C10 and C13. A chemical group (ketone, hydroxyl…) or an alkyl side-chain may also be present at C17. Steroids may possess a nucleus derived from sterane by one or more C-C bond scissions or ring expansions or contractions.
The term “steroids” was coined by Callow RK et al. (Proc Royal Soc London series A 1936, 157, 194) “for the group of compounds comprising the sterols, bile acids, heart poisons, saponins, and sex hormones“.
Several steroid derivatives with aromatic ring(s), steroid phosphate esters derived from marine invertebrates, and steroids incorporating halogen atoms (I, Br, or Cl) have been described (review in: Dembitsky VM, Molecules 2023, 28, 5549). These compounds are either produced by fungi or fungal endophytes or found in extracts of plants, algae, or marine invertebrates.
As natural steroids are derived from squalene by cyclization, unsaturation and substitution, they may be considered as modified triterpenes. Fatty acid esters of steroids are found mainly in the blood but their exact role is not known to date. An efficient analytical method for the simultaneous determination of 12 esters in serum has been desscribed (Jung HJ et al., J Chromatogr A 2009, 1216, 1463).
There is a close connection between modern-day biosynthesis of particular triterpenoid biomarkers and presence of molecular oxygen in the environment. Thus, the detection of steroid and triterpenoid hydrocarbons far back in Earth history has been used to infer the antiquity of oxygenic photosynthesis (Summons RE et al., Phil Trans R Soc B 2006, 361, 951). A great variety of sterol structures are found within the marine environment, many of which are only known to be biosynthesized by a limited number of organisms. This, coupled with the comparatively high resistance of the sterol skeleton to extensive degradation, makes them valuable as biological markers (Philp R P et al., Sci Progr, Oxford, 1976, 63, 521). Steroid acids are unique molecular fossils composed of a steroid carbon skeleton linked to a carboxyl group. These biomarkers contain special geological and biological clues in their structures.
The study of sterol distribution in a marine sediment may indicate the likely source organisms which contributed the organic matter to the sediment. Results have suggest that microbial activities indeed contribute to the formation of carboxyl groups on the eukaryotic steroid skeletons (Xie C et al., Org Geochem 2024, 194, 104816),
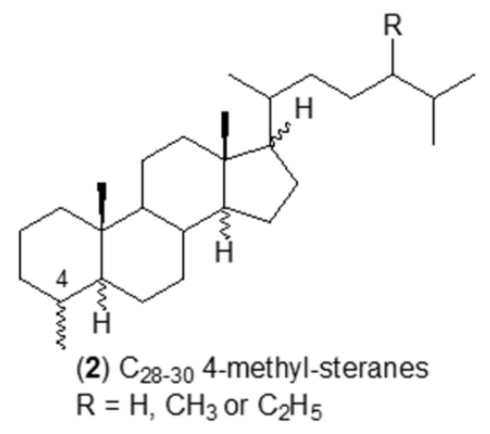
3-Methyl steroids have not been reported in living organisms but were discovered in sediments and oils and some immature source rock bitumens (Killops SD et al., Chem Geol 2022, 607, 121014). It seems possible that they are produced by a limited degree of rearrangement of steroids after they are bound into kerogen or upon cleavage from kerogen and methylation during catagenesis.
Numerous studies have identified several saturated steroidal hydrocarbons (i.e. steranes) in oils and rock extracts: C21–C26 20-n-alkylpregnanes, C28–C30 4-methyl steranes were reported in Tertiary lacustrine sediments (Chen J et al., Org Geochem 2001, 32, 115), C24 and C25 4,4,14-trimethyl steranes in Tertiary sulfur-rich crude oils (Lu H et al., Org Geochem 2011, 42, 146), 3β-alkyl steranes in Eogene lacustrine sediments (Wang G et al., Chin Sci Bull 2006, 51, 1628), and 3β-methylcholestanes in the Neoproterozoic-Cambrian Salt Basin (Grosjean E et al., Org Geochem 2009, 40, 87). Specific sterane signatures have been used as diagnostic for eukaryotic organisms. For example, 4-methyl steranes are related to dinoflagellate contributions to the organic matter. Cholestanes, 24-methylcholestanes and 24-ethylcholestanes are also related to the contribution of distinct organisms, indicating a particular depositional environment. The presence of 4-methyl steranes was reported in conifer fossils for the first time, pointing toward another likely origin from higher plants (Zhan X et al., Appl Geochem 2022, 143, 105328).
Several chlorine containing steroids are present in plants. Thus, the first chlorine-containing steroids, jaborosalactone C and jaborosalactone E, were isolated from the leaves of the Jaborosa integrifolia plant (family Solanaceae) (Tschesche R et al., Tetrahedron.1968, 24, 5169). Later, several others have been isolated from various solanaceae species (review in Dembitsky VM et al., Int J Curr Res Biosci Plant Biol 2017, 4, 70). Their roles for drug discovery and their great interest to chemists, physicians, biologists, pharmacologists and the pharmaceutical industry are also reviewed.
According to their chemical structure, the wide array of steroid molecules may be divided into several groups :
Sterols
Brassinosteroids
Bufadienolides
Cardenolides
Cucurbitacins
Ecdysteroids
Sapogenins
Steroid alkaloids
Withasteroids
Bile acids
Furanosteroids
Hormonal and neuronal steroids
![]()
They are derivatives of cholestane with two vicinal diols (C-2, C-3 and C-22, C-23) and a 6-keto group.
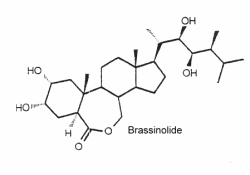
They are a unique class of plant growth regulators with structural similarity to animal steroid hormones, and ecdysteroids from insects and crustacea. Many of them may be considered as sterols. Brassinolide is the first biologically active compound isolated from the pollen of Brassica napus in 1979 (Grove MD et al., Nature 1979, 281, 216) after reporting stem elongation and cell division by the treatment with organic extracts of rapeseed pollen. It has been reported an epigenetic mechanism that governs brassinosteroid biosynthesis and orchestrates the expression of specific genes to modulate brassinosteroid levels and influence rice growth and development (Zhang Y et al., J Agric Food Chem 2024, 72, 19629).
Over 60 analog compounds have been isolated but brassinolide exhibits the highest biological activity of the known brassinosteroids.
As it was shown that these compounds could be potent plant growth and development regulators, dozens of compounds of similar structure were isolated from plant sources (algae, ferns, gymnosperms, and angiosperms, but not bacteria) or synthesized. It was shown that they interact with jasmonates in the formation of anti-herbivory traits in tomato (Campos ML et al., J Exp Bot 2009, 60, 4347).
Extensive reviews on brassinosteroids released by Zullo MAT and Clouse SD may be consulted with interest. The complex role of brassinosteroids in plant developmental and physiological responses has been reviewed (Gudesblat GE et al., Curr Opinion Plant Biol 2011, 14, 530). It has been demonstrated that brassinosteroids also confers freezing tolerance in plants (Eremina M et al., PNAS 2016, 113, E5982). Extensive studies have demonstrated that brassinosteroids signaling is involved in plant innate immunity as well as the response to environmental stimuli including extreme temperatures, saline-alkali, and drought (Yao T et al., J Agric Food Chem 2023, 71, 7947). Advances implementing high-resolution and modeling tools that have provided new insights into the role of brassinosteroid signaling in growth coordination across cell layers have been reviewed (Aardening Z et al., Trends Plant Sci 2025, 30, 389).
They are typically polyhydroxy C24 steroids with a pentadienolide ring at C-17. The structure of hellebrigenin is given below as a typical example of bufadienolide
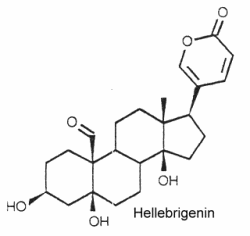
They have been isolated from plants and animals. More than 250 compounds have been identified. In plants, thay are mostly glycosides with one to three sugars in a chain linked to the 3-hydroxyl group.
They are important for their cardiotonic activity. Furthermore, they possess insecticidal and antimicrobial properties, those produced by the toad skin are strongly poisonous.
An extensive review on bufadienolides released by Steyn may be consulted with interest.
Their structure is closely related to bufadienolides but these C23 steroids possess a butenolide ring located at C-17. The structure of digitoxigenin is given below as a typical example of cardenolides.
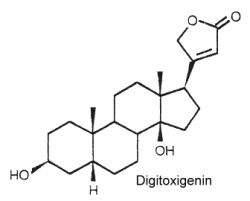
They are widely distributed in plants mainly as glycosides and are either toxic or insect deterrents. As potent cardiotonics, through their inhibition of Na/K- ATPases, these steroids were largely studied (digoxin and its derivative ouabain…). Monarch butterfly is well known to be highly toxic to birds because of cardenolides which come from the milkweed leaves eaten by its caterpillar. Experimentally, the larvae of the lepidopteran Trichoplusia ni were poisoned by feeding on the milkweed Asclepias curassavica, which contains cardenolides in its latex (Dussourd DE et al. Chemoecology 2000, 10, 11).
They are the most oxygenated C30 triterpenoids with a dimethyl group at C-4 and methylated at C-9 and C-14. Strictly, they are not steroid since they are not methylated at C-10. The structure of cucurbitacin D is shown below as a typical example of cucurbitacins.
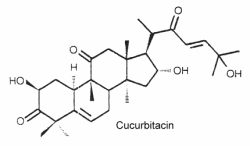
These steroids which are commonly combined in glycosides, are mainly associated with cucurbitaceae species (including the common pumpkins and gourds) but they have also been detected in other families, even in mushrooms and in some marine mollusks. About 50 species have been identified.
Mammals perceived these toxic molecules as some of the bitterest substances known, they give a bitter taste in plant foods such as cucumber, zucchini, melon and pumpkin. Some cucurbitacins have been shown to have a prominent antitumor activity.
They have protective effects against herbivores but are feeding stimulants for some beetles. Cucurbitacins have been shown to act as ecdysteroid receptor antagonists.
These C27 steroids have in common a 7-en-6-one chromophore, sometimes a methyl group at C-24 and several hydroxyl groups increasing their polarity.
Ecdysteroids are a class of invertebrate steroid hormones, first found in insects, in which they regulate activities such as molting, development, and reproduction, including the critical metamorphic phases in arthropods. The first ecdysteroid, ecdysone, was isolated from silkworm pupae by Butenandt and Karlson in 1954, and its structure was presented in 1965 by Huber and Hoppe. Nowadays, over 550 ecdysteroids are known.
The structure of ecdysone is given below as an example of these steroids. Its structure was first elucidated from a hormonal fraction extracted from silk worm pupae (Karlson P, Naturwissenschaften 1966, 53, 445).
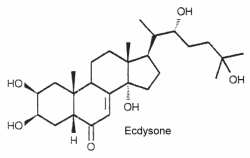
Ecdysteroids are present both in animals (arthropods) and plants. About 500 species have been identified (review in: Savchenko RG et al., Phytochem Rev 2022, 21, 1445).
Ecdysteroids are classified into three main groups based on their natural origin, including phytoecdysteroids, zooecdysteroids, and mycoecdysteroids (Arif Y et al., Int J Mol Sci 2022, 23, 8664).
Phytoecdysteroids seem to protect plants against most insects. Most phytoecdysteroids possess a cholest-7-en-6-one carbon skeleton and a hydroxyl group on the C14. The carbon number can vary between C19-C29 (sometimes C30).
They occur in a variety of plant families, including Asteraceae, Amaranthaceae, Commelinaceae, Liliaceae, Lamiaceae, Magnoliaceae, Podocarpaceae, Ranunculaceae, etc. Representative species of plant families include Ajuga turkestanica, Rhaponticum carthamoides, Pfaffia glomerata, Cyanotis arachnoidea, etc.. These plants have been used as adaptogens, antioxidants, tonifying agents, and to promote muscle growth and strength since ancient times.
Among many structures, it can be noticed the presence of ajugalactone in Ajuga reptans, ajugasterone C in Vitex madiensis, cyasterone in Ajuga chamaepitys, inokosterone in Achyranthes fauriei, makisterone B in Ajuga chamaepitys, ponasterone A in Podocarpus nakaii , polypodine B in Polypodium vulgare and poststerone in Cyathula capitata. Insects that ingest phytoecdysteroids and are not adapted to this defense are subject to serious adverse effects, including reduced weight, molting disruption, and/or mortality.
In insects, precursors are produced by prothoracic glands and metabolites are known to trigger a cascade of morphological changes through specific receptors (molting hormones). The most efficient is 20-hydroxyecdysone. Relationships between plants and insects have been hypothesized. It appears that all arthropods employ essentially the same compound as the molting hormone.
Asparagus (Asparagus officinalis), known as the “king of vegetables”, has been identified as a source of 20-hydroxyecdysone, particularly in its hard stem by-product, which contains relatively high levels of that compound, approximately 2.34 mg/g dry weight (Denben B et al., Sports 2023, 11, 175).
An extensive data base may be consulted with interest.
In humans, 20-hydroxyecdysone and other ecdysteroids are marketed as ingredients in nutritional supplements for various sports, particularly bodybuilding. Evidence to support such use, however, is limited. A comprehensive study, designed to find any strength or athletic improvement from 20-hydroxyecdysone, was published in 2006 and concluded that using 30 mg per day of 20-hydroxyecdysone administered orally did not significantly affect anabolic or catabolic responses to resistance training, body composition, or training adaptations (Wilborn C.D. et al., J Int Soc Sports Nutr 2006, 3 (2): 19). New data seem to prove the effectivity of an ecdysterone supplementation with respect to sports performance. It appears that ecdysterone and turkesterone are compounds with prominent potential in sport and healthy nutrition (Todorova V et al., Nutrients 2024, 16, 1382). Findings have demonstrated that 20-hydroxyecdysone supplementation is a promising way to maintain muscle mass and strength during detraining. Accordingly, it may prevent muscle mass and strength loss due to detraining by lowering catabolic hormone levels (Denben B et al., Sports 2023, 11, 175)..
These results strongly suggest the inclusion of ecdysterone in the list of prohibited substances as other anabolic agents (Isenmann E. et al., Arch Toxicol (2019).
They form the aglycon part of saponins which have well known detergent properties. They are oxygenated C27 steroids (phytosteroids) with an hydroxyl group in C-3. The structure of diosgenin is given below as an example of these compounds. Similar compounds are: dioscin, neoruscogenin, ruscogenin, and sarsasapogenin. They belong to the spirostan class of phytosteroid and are characterized by a unique spiro ring structure in their chemical composition, composed by cross-bonded furan and pyran rings. Spirostan-type phytosteroids are commonly found in various plant species, such as fenugreek, yam, yucca and some species of Solanum, usually as glycosides. In such conjugated form, they are often named « steroidal saponins ». These compounds have been studied for their potential health benefits,
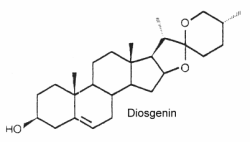
These steroids can mimic or regulate steroid hormones. Thus, diosgenin can be chemically converted into corticosteroids, estrogens and progesterone. They are externally distributed in plants since they occur in over 90 plant families. They are used in nutrition, as herbal medicine, and in cosmetics. The cytotoxic and antimicrobial activities of steroidal saponins from Yucca rostrata and Dracaena braunii were reported (Nguyen DH et al., Biochem Système Ecol 2024, 113, 104791).
In nature, commonly found saponins consist of (25S)-spirostan derivative (known as real saponins or neosaponins), while (25R)-spirostan derivatives are also known as isosaponins.
They form a large group of molecules where a nitrogen atom is integrated into a ring or in a substituent. The steroid nucleus can contain double bonds and hydroxyls in various positions. The structure of solasodine found in chili and paprika is given below as an example of these compounds.
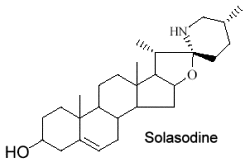
These alkaloids are only distributed in Solanaceae (potato, tomato, eggplant …). Fortunately, their toxic properties disappear by structural transformation during ripening. Solasodine is the most common species in Solanum.
The steroidal alkaloids may be broadly classified into two major groups (Pharmacognosie), namely:
1- Solanum Alkaloids : Solanaceae accumulate several steroidal alkaloids based on a C27 cholestane skeleton, such as: solasodine, tomatidine, solanidine. They are found in Solanum laciniatum; S. dulcamara; S. nigrum; S. torvum; S. lycopersicum; S. tuberosum; S. aviculare etc. These alkaloids normally occur as their corresponding glycosides (thus, solasonine contains solasodine linked to rhamnose, glucose and galactose).
2- Veratrum Alkaloids : They represent the most important and medicinally significant class of steroidal alkaloids. The basic ring systems present in the Veratrum alkaloids are characterized by the ring ‘C’ is a five-membered ring while ring ‘D’ is a six-membered ring which apparently is just the reverse of the pattern in the regular steroidal nucleus as present in cholesterol. The important alkaloids belonging to this group of alkaloids are: protoveratrines; veratridine, cevadine, germine etc.
They typically C28 ergostane-type steroids with a 22,26-lactone.They are also characterized by a large number of oxygenated functions (hydroxyls, ketones, epoxides …). 90% of withasteroids (or withanolides) possess a 1-oxo-group as shown below in withaferin.
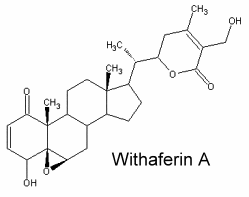
Over than 900 species have been described, some of them as glycosides. They are predominantly associated with Solanaceae but are also found in other families (Taccacceae, Leguminosae, Labiatae). Withanolides are known to have important pharmacological properties but they are also antimicrobial, insect deterrent or ecdysteroid receptor antagonists. They have been used, and in particular those extracted from Withania somnifera roots (ashwagandha) for over 3,000 years in traditional Ayurvedic and Unani Indian medical systems. Traditionally, the extracts were ascribed a wide range of pharmacologic properties with corresponding medical uses, including adaptogenic, diuretic, anti-inflammatory, sedative/anxiolytic, cytotoxic, antitussive, and immunomodulatory (White PT et al., Adv Exp Med Biol. 2016, 928, 329).
The end products of cholesterol utilization are the bile acids, synthesized in the liver. In mammals, the most common bile acids are C24 steroids with a carboxyl group at C-24 and up to three hydroxyl groups on the steroid nucleus, one being at C-3. The most abundant bile acids in human bile are chenodeoxycholic acid (45%) and cholic acid (31%). These are referred to as the primary bile acids. Within the intestines the primary bile acids are converted by bacteria into the secondary bile acids, identified as deoxycholate (from cholate) and lithocholate (from chenodeoxycholate). These compounds are reabsorbed by the intestines and delivered back to the liver via the portal circulation. Within the liver the carboxyl group of primary and secondary bile acids is conjugated via an amide bond to either glycine or taurine before their secretion into the bile. These conjugation reactions yield glycoconjugates and tauroconjugates, respectively. They are hydrolized in the intestine. Glycoconjugates are present among eukaryotes only in mammals, but they were also detected (with deoxycholic acid) in a marine bacterium, Myroides sp. (Maneerat S et al., Appl Microbiol Biotechnol 2005, 67, 679).
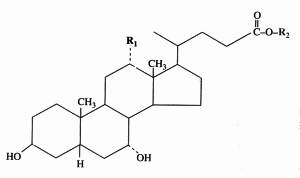
Cholic acid : R1 = OH, R2 = H
Chenodeoxycholic acid : R1 = R2 = H
Glycocholic acid : R1 = OH, R2 = NH-CH2-COOH
Taurocholic acid : R1 = OH, R2 = NH-CH2-CH2-SO3H
Bile acids have long been known to be essential in dietary lipid absorption and cholesterol catabolism. Furthermore, an important role for bile acids as signaling molecules has emerged. They were shown to activate mitogen-activated protein kinase pathways, to be ligands for the G-protein-coupled receptor TGR5 and to activate nuclear hormone receptors (Houten SM et al., EMBO J 2006, 25, 1419). Bile acids have been discovered to activate specific nuclear receptors (FXR, PXR, Vitamin D receptor), a cell surface receptor (TGR5), and cell signaling pathways (JNK 1/2, AKT and ERK 1/2) in cells in the liver and gastrointestinal tract (review in Hylemon PB et al., J Lipid Res 2009, 50, 1509). Several works provide evidences of bile acid signaling in regulation of glucose and lipid metabolism (Li T et al., J Lipids 2012, ID 754067).
Several 3-keto-cholestenoic acids (dafachronic acids) were shown to be involved in the control of dauer formation and reproduction in the nematode Caenorhabditis elegans (Motola DL et al., Cell 2006, 124, 1209). Investigations revealed that the nuclear hormone receptor DAF-12 from that worm was optimally activated by two isomers, (25S)-D7- and (25S)-D4-dafachronic acid with EC50 values of 23 and 33 nM, respectively (Sharma KK et al., Mol Endocrinol 2009, 23, 640). One of these isomers is shown below.
(25S)-D7-Dafachronic acid
It has been shown that bile acids were able to function as nutrient signaling molecules primarily during the feed/fast cycle as there is a flux of these molecules returning from the intestines to the liver following a meal
induce. They regulate the increase in energy expenditure in brown adipose tissue, preventing obesity and resistance to insulin (Watanabe M et al., Nature 2006, 439, 484). This novel metabolic effect is dependent on induction of the cyclic-AMP-dependent thyroid hormone activating enzyme type 2 iodothyronine deiodinase.
It was shown in Caenorhabditis elegans that a dietary restriction or starvation leads to an increased production of that steroid that is also required, but not sufficient, for the diet-mediated longevity enhancement (Thondamal M et al., Nat Commun 2014, 5:4879).
A simple and rapid procedure for the isolation of bile acid fraction using a solid-phase extraction on a C18 column has been described (Persson E et al., J Lipid Res 2007, 48, 242).
Some types of bile acids (e. g., (isolithocholic acid)) can be esterified with long-chain fatty acids (e.g., palmitic acid, palmitoleic acid, stearic acid, and oleic acid) (Kelsey MI et al., J Lipid Res 1980, 21, 751). Thus, more than 50 secondary bile acids and their conjugated forms have been found in human feces (Ridlon JM et al., J Lipid Res 2006, 47, 241). A study provided insight into the establishment of early gut microbiota and the interactive development of esterified bile acids (Takei H et al., J Lipid Res 2022, 63, 100275).
Conjugates of fatty acid with bile acids are a new class of molecules synthesized with the aim of reducing cholesterol crystallization in bile. Among them, arachidyl amido cholic acid (Aramchol) was shown to be the most active to retard that process and may be of potential use in cholesterol gall stone disease in humans (Gilat T et al., Gut 2001, 48, 75).
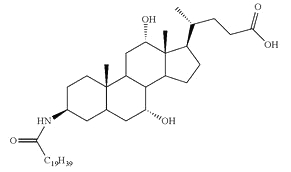
Aramchol : Arachidyl amido cholic acid
Aramchol has been shown to activate cholesterol efflux by strongly stimulating the ABCA1 transporter, a universal cholesterol export pump present in all cells (Leikin-Frenkel A et al., Arch Med Res 2010, 41, 6). In animal models, this led to a significant reduction of blood and body cholesterol and an increase in fecal sterol output, mostly neutral sterols.
A documented review on bile acids, present in all vertebrates except fish and bile alcohol present in fish, may be consulted (Hofmann AF et al., Cell Mol Life Sci 2008, 65, 2461).
Bile acids determination : As early as 1974, an almost complete separation of bile acids was done by TLC (Fausa O et al., Scand J Gastroenterol 1974, 9, 567). Analyses using HPLC coupled with refractive index detector or with ultraviolet detection were proposed, but their low sensitivity limited their applications. More recently, it has been shown that evaporative light-scattering detection improved both selectivity and sensitivity (Persson E et al., J Lipid Res 2007, 48, 242). A further improvement was observed using an isocratic HPLC charged aerosol detector which enabled the determination of individual bile acids in human gastric and duodenal aspirates (Vertzoni M et al., J Lipid Res 2008, 49, 2690).
FURANOSTEROIDS
Furanosteroids represent a class of pentacyclic isoprenoid lipids that were identified in mushrooms and in various other organisms. They possess additional fused furan, dihydrofuran, or tetrahydrofuran ring(s) attached to their pentacyclic backbone. That extra furan ring is connecting positions 4 and 6 of the steroid skeleton. The term “furanosteroids” has been expanded to include any steroids and related compounds that contain the furan ring, 2,3-dihydrofuran ring, 2,5-dihydrofuran ring, and/or tetrahydrofuran ring (s). Several metabolites belonging to this lipid class have potent anti-inflammatory and antibiotic properties, potential anti-proliferative activity, and also exhibit activity in inhibiting protein kinase C, phospholipase A2, and eliciting cytotoxicity against cancer cells (review in : Dembitsky VM, Molecules 2023, 28, 10.3390).
As an example, Viridin, is an antibiotic produced by a strain of the common soil fungus Trichoderma viride isolated in 1945. It is not antibacterial but is highly antifungal, its activity against certain fungi being remarkably high. Furanosteroids, their analogues and derivatives, comprise a collection of over 1000 compounds (Buckingham, J. Dictionary of Natural Products; CRC Press: Boca Raton, FL, USA, 2022).
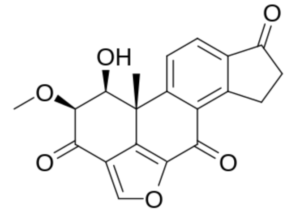 Viridin
Viridin
This large group can be divided into three major families, mainly on the basis of their physiological function or their tissue origin : the sexual hormones, the corticosteroids, and the neurosteroids.
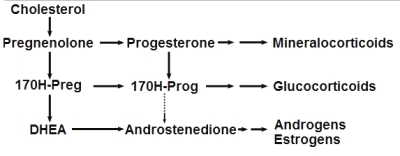
Steroidogenesis pathway
(from Wikipedia Commons)
While best known in vertebrates, the presence of several steroids in aquatic invertebrates (echinoderms, molluscs, and crustaceans) have been already reported (Janer G et al., Ecotoxicology 2007, 16, 145). However, these results obtained by immunoassay methods should be regarded with caution, because of possible cross-reactivity and interferences (Devier MH et al., Anal Chim Acta 2010, 657, 28).
Pregnane is the parent of progesterone, ketones, and several adrenocortical hormones. It is found largely in urine as a metabolic product of 5ß-pregnane compounds. It is found also in ancien sediments and petroleum.
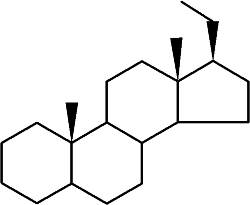
Pregnane
While the abundance of pregnane isomers relative to regular steranes increases in oil with increasing thermal maturity, this was attributed to differences in thermal stability. It was suggested that pregnane compounds originate from kerogen-bound sterol precursors during diagenesis and catagenesis (Wang G et al., Org Geochem 2015, 78, 110). They represent novel indicators in exploration of ancient sediments and petroleum.
This important group may be again divided into estrogens, progestagens, and androgens. It must be noticed that androstenedione, produced in the adrenal glands and the gonads (ovary and testicles), is the common precursor of all sexual hormones (see Steroidogenesis pathway). In females, androstenedione is produced by theca cells and exported in granulosa cells for estrogen production. This steroid has a function of sexual pheromone in fish (Sorensen PW et al., Gen Comp Endocrinol 2005, 140, 164).
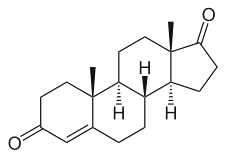
Andostenedione
This compound was also detected in the pollen of a pine species (Pinus sylvestris) (Saden-Krehula M. et al., Experientia 1971, 27, 108). It has been also reported in Nicotiana tabacum and Inula helenium at a level of 8 to 11 pmol/g (Simersky R et al., J Plant Growth Regul 2009, 28, 125). Its presence is also documented in waters and bottom sediments of rivers which receive paper mill effluent.
Estrogens : They are C18 steroids generally with a phenolic function at C-3 (the first ring A being aromatic), without methyl group at C-10, and with always an oxygenated function at C-17. 17β-estradiol is the model molecule.
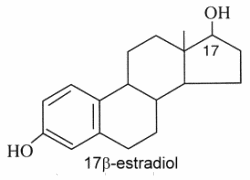
Estrone (or folliculin) is a compound similar to 17β-estradiol but with a ketone group on the C-17. This hormone was discovered by Butenandt A (Nobel Prize in chemistry, 1939). Secreted by the ovary, it has estrogenic activity but is also present in plants (pollen and seeds of date palm, seeds of pomegranate). Pollen of the date palm has been used for long time as a traditional Egyptian herbal medicine for improving male and female fertility, a property likely related to the presence of estrone besides other sexual hormones (Tatar T et al., J Am Coll Nutr 2018, 37, 154).
The glucuronide derivative of estradiol has been found in high concentrations in seawater around spawning Euphyllia ancora (a stony coral) seawater, and has therefore been implicated as a candidate signaling molecule in spawn synchronisation (Twan WH, et al., Fish Physiol Biochem, 2005, 31, 111). It seems thus evident that Corals already evolved the vertebrate-type hormone system in their sexual reproduction.
Phytoestrogens are types of estrogens not generated within the endocrine system, but consumed by eating plants or manufactured foods. These “dietary estrogens” with questionable lipid properties are a diverse group of non steroidal plant compounds which have the ability to cause estrogenic or antiestrogenic effects because of their structural similarity to estradiol. Among them, coumestans, prenylflavonoids and isoflavones are the most active in estrogenic effects in this class, isoflavones being the best-researched. Numerous health benefits have been described including reproductive health, heart health, a role in weight loss, hormone-dependent tumours, bone and skin health, and the immune system (Canivenc-Lavier MC et al., Nutrients 2023, 15, 317).
Progestagens : They are C21 steroids with a en-4-one-3 group and a ketone function at C-20. Progesterone is the model molecule. In 1934, Butenandt A (Nobel Prize in chemistry, 1939) and Westphal U succeeded in producing this hormone in a chemically pure form. The establishment of its ovulation-inhibiting properties following animal experiments was conducted up to 1939.
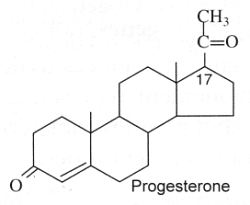
High levels of progesterone have been reported in plants, Nicotiana tabacum and Digitalis purpurea (55 to 59 pmol/g) (Simersky R et al., J Plant Growth Regul 2009, 28, 125).
Guggulsterone is an analogue of progesterone. That sterol is found in the resin of the guggul tree (Commiphora mukul).
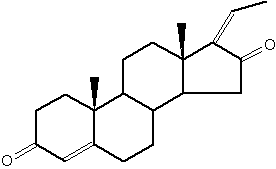
Guggulsterone
Guggul tree extract has been suggested to lower low-density lipoprotein levels in animal models, it has been successfully used in Ayurveda medicine since at least 600 BC to treat obesity and lipid disorders (Satyavati GV et al., Indian J Med Res 1988, 87, 327; Singh V et al., Pharmacol Res 1990, 22, 37).
It has been also shown that guggulsterone is an efficacious antagonist of the liver X receptor (FXR) and the bile acid receptor (Urizar NL et al., Science 2002, 296, 1703); Wu J et al., Mol Endocrinol 2002, 16, 1590). It has been proposed that inhibition of FXR activation is the basis for the cholesterol-lowering activity of guggulsterone. Later, it was demonstrated that no overall reduction in total cholesterol and levels of low-density lipoprotein occur after guggulsterone administration in humans (Szapary PO et al., JAMA 2003, 290, 765).
Sulfate and ester derivatives of guggulsterone have been proposed as component of nanosomes or liposomes for drug delivery (Ahmad MU et al., Chem Phys Lipids 2010, 163, 362).
Norethisterone was synthesized in 1951 and was one of the first progestins to be developed. It was first introduced for medical use in 1957 and was introduced in combination with an estrogen for use as a birth control pill in 1963 (Wikipedia). That was the first generation of contraceptive pill.
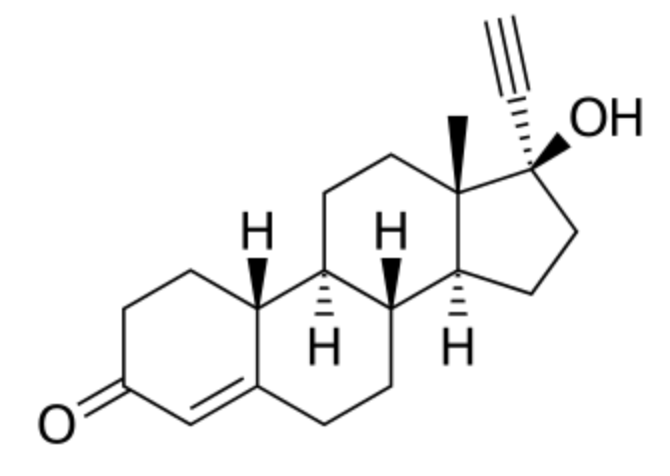
Norethisterone
Androgens : They are C19 steroids. The major androgen is testosterone which is a 17β-hydroxysteroid with a en-4-one-3 group. This steroid was also detected in the pollen of Scots pine (Pinus sylvestris).
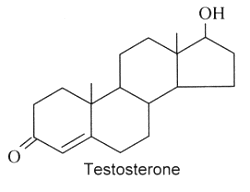
Several testosterone derivatives are present in human male secretions such as sweat, saliva, and semen and have been implicated as putative human pheromones. Among them, the most studied is androstadienone (Grosser BI et al., Psychoneuroendocrinology 2000, 25, 289). It was shown that smelling androstadienone was able to maintain higher levels of cortisol in women (Wyart C et al., J Neurosci 2007, 27, 1261).
Androstenol and androstenone were also characterized in human sebum.
|
|
|
|
Abiraterone is a potent androgen synthesis inhibitor which is used in combination with prednisone in metastatic castration-resistant prostate cancer. It was approved by the United States Food and Drug Administration in 2011.
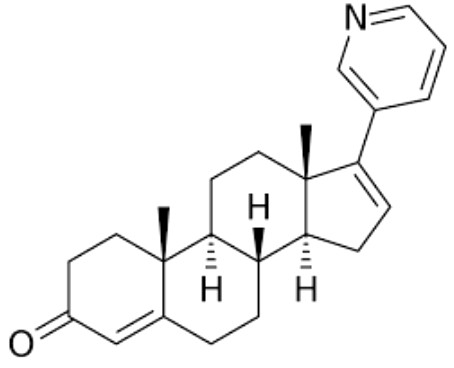
Abiraterone
Theoretically, these compounds, named also corticoids, should be formed in the adrenal cortex. Furthermore, they must be C21 steroids and three or more oxygen atoms. They have all a en-4-one-3 group and an oxygenated function at C-20. The major corticosteroids in vertebrates are cortisol which has an hydroxyl group at C-11, C-17, and C-21 (glucocorticoid hormone) and aldosterone which has only one hydroxyl group at C-11 and one aldehyde function at C-18 (mineralocorticoid hormone).
|
|
|
Corticosteroid hormones in vertebrates are critical for metabolism, growth, reproduction, immunity, and ion homeostasis, and are an important part of the coping mechanisms involved in the stress responses. In tetrapod groups, there are at least two active glucocorticoid hormones, either cortisol or corticosterone, and one mineralocorticoid hormone, aldosterone, which regulates ion balance. In contrast, in teleosts, cortisol apparently has both activities, whereas aldosterone is not present. It has been clearly determined that 11-deoxycortisol (one of the precursors of cortisol) is the only corticosteroid hormone present in the earliest vertebrates, the agnathans (the lamprey) (Close DA et al., PNAS 2010, 107, 13942).
Recent discoveries have revealed that brain is a site of extensive steroid metabolism and also a target of steroid hormones. These natural or synthetic steroids rapidly alter the excitability of neurons by binding to membrane-bound receptors such as those for inhibitory and (or) excitatory neurotransmitters. These hormones play an important role in the development, growth, maturation and differentiation of the brain (Baulieu EE, Psychoneuroendocrinol 1998, 23, 963). The term “neurosteroid”, proposed by EE Baulieu in 1981, applies to steroids which are accumulated in the brain independently of supply by peripheral endocrine glands and which are synthesized from cholesterol in the nervous system. Several steroids have been described in the brain since the first report in 1981 of dehydoepiandrosterone (DHEA) and its sulfated derivative in the rat brain (Corpechot C et al., PNAS 1981, 78, 4704). Among the best known are pregnenolone, progesterone, allopregnanolone and DHEA. A review of the pleiotropic and protective abilities of neurosteroids and hormonal steroids may be consulted (Melcangi RC et al., Cell Mol Life Sci 2008, 65, 777).
Numerous investigations have been focused on a series of sedative-hypnotic 3 alpha-hydroxy ring A-reduced pregnane steroids that include the major metabolites of progesterone and deoxycorticosterone, 3 alpha-hydroxy-5 alpha-pregnan-20-one (allopregnanolone) and 3 alpha,21-dihydroxy-5 alpha-pregnan-20-one, (allotetrahydroDOC), respectively. These 3 alpha-hydroxysteroids do not interact with classical intracellular steroid receptors but bind stereoselectively and with high affinity to receptors for the major inhibitory neurotransmitter in brain, gamma-amino-butyric acid (GABA) (review in: Paul SM et al., FASEB J 1992, 6(6), 2311). Thus, receptor-active neurosteroids may represent an important class of neuromodulators that can rapidly alter central nervous system excitability via novel nongenomic mechanisms.
Pregnenolone is the product of cholesterol conversion by a P450 oxydase complex (cholesterol side-chain cleavage enzyme, P450scc) and is the immediate precursor of progesterone. This steroid is found in free form or as a sulfated derivative. Its function is mainly a negative modulation of GAGA-A receptor activity and a positive modulation of NMDA receptors. Several studies suggest that pregnenolone plays an important role in the control of neural development and in the improvement of neuron plasticity.
|
|
A derivative of pregnenolone was synthesized by Aelis Pharma, AEF0117 (3β-(4-methoxybenzyloxy)pregn-5-en-20-one), which is a biased allosteric modulator of the cannabinoid CB1 receptor, representing a new class of compounds referred to as CB1-selective signalling-specific inhibitors (CB1-SSi). Preliminary clinical trials in patients diagnosed with cannabis use disorder, have shown that he was able to partly but not completely block the effects of tetrahydrocannabinol (THC), thus reducing cannabis self-administration (Haney M et al., Nature Med 2023).
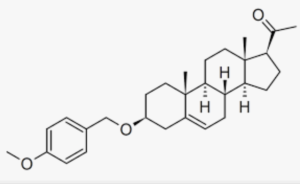
AEF0117
It was previously demonstrated that in response to high doses of THC, a hormone, pregnenolone, was synthesized, thus reducing some of the effects of THC (Vallée M et al., Science 2014, 343, 94).
Progesterone, as described above is a progestagen, but also is active at the brain level. That important steroid is formed directly from pregnenolone in neurons and glial cells by a 3b-hydroxysteroid dehydrogenase. Its sedative and anesthetic properties have been described as soon as 1941 by H. Selye. Among its numerous functions, it can be noticed that progesterone has important consequences for myelinisation, neuronal development, survival and regeneration of the nervous system.
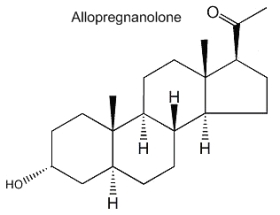
Allopregnanolone (3a-hydroxy-5a-pregnan-20-one) is formed from progesterone by the action of 5a-reductase and 3b-hydroxysteroid dehydrogenase. It acts mainly in modulating the GABA-A receptor activity and its physiological role is important in neurogenesis, survival and migration of neurons. Furthermore, its involvement in the stress reaction suggests that this neurosteroid could have implications in depressive disorders.
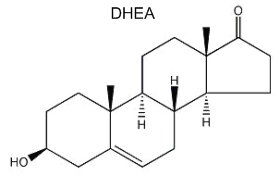
Dehydroepiandrosterone (DHEA) is the first neurosteroid discovered in a mammalian brain and, thus, is the most actively studied. DHEA is a direct metabolite of pregnenolone and is found in free form or as a sulfated derivative. The great interest in this neurosteroid is the observation of its abundance in human brain and blood and its concentration lowering during stress situations and aging. Recent studies amphasize its role in neurogenesis, survival and protection of neuronal cells.
Devenez membre et participez au développement de la Lipidomique au XXIème siècle.
S'inscrire