
They are the terpenes that have been known for several centuries as components of the fragrant oils obtained from leaves, flowers and fruits. They are produced from the assembly of two isoprene units (C10), constituting about 90% of plant essential oils and permitting a very large variety of chemical structures (around 1000 metabolites). According to their structural variation, monoterpenes can be divided into different subgroups: acyclics, bornanes, camphanes and isocamphanes, fenchanes, thujanes, p-menthanes, and caranes and pinanes. While a few, such as camphor, occur in a near pure form, most occur as complex mixtures, often of isomers difficult to separate. These essential oils have numerous actions, such as allelochemical functions between plants and between plants and predators. A role in wound healing was also observed. An article has reviewed publications and patents concerning the pesticidal activities of frequently studied monoterpenes and their modes of action (Liu Z et al., J. Agric. Food Chem. 2022, 70, 15, 4556).
It is now admitted that boreal forests modify atmospheric particles in emitting a large amount of monoterpenes, a large proportion of these biogenic volatile organic compounds are oxidized by ozone in the atmosphere. They form products with low enough vapour pressure to condense on existing aerosol particles, forming secondary organic aerosol, their strength of emission depending on the tree species and varying according to temperature and light among other variables. Through these productions, forests impact regional and global climates (Spracklen D et al., Phil Trans R Soc A 2008, 366, 4613). In the boreal region of northern Europe, monoterpenes have concentrations ranging from some tens of parts per trillion up to parts per billion (Tunved P et al., Science 2006, 312/5771, 261).
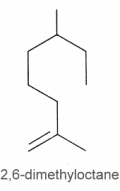
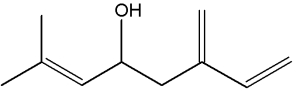
They can be considered as derivatives of 2,6-dimethyloctane.
Among natural molecules, the followings are well known and have several structural isomers.
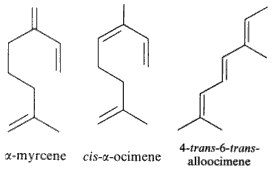
Myrcene is produced mainly semi-synthetically from Myrcia (Myrtaceae) and Thymus (Lamiaceae). It is an intermediate in the production of several fragrances. It is often produced commercially by the pyrolysis of β-pinene, Many other plants contain myrcene, some of these include Cannabis, hops, Houttuynia, lemon grass, mango, Schinus molle, bay leaf (Laurus nobilis) and cardamom. Myrcene is thought to be also the predominant terpene found in modern Cannabis cultivars within North America. Its antinociceptive effect was demonstrated for the first time in mice suggesting a mediation by α2-adrenoreceptor stimulated release of endogenous opioids (Rao VSN et al., J Pharm Pharmacol 1990, 42, 877).
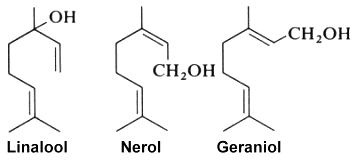
Linalool (or linalol) is well known as it is naturally present in almost all essential oils and especially those of coriander (Coriandrum sativum L.), cymbopogon (Cymbopogon martini var. martinii), sweet orange (Citrus sinensis), lavender (Lavandula officinalis), bay laurel (Laurus nobilis), and sweet basil (Ocimum basilicum), among others. It was first synthesized in the laboratory of Leopold Ružička in 1919, joint winner of the 1939 Nobel Prize in Chemistry.
Colorless, it brings a floral and fresh touch. Some will say that its smell is similar to that of lily of the valley. It is notably the precursor of linalyl acetate, widely synthesized to obtain the aroma of lavender. With an allergenic reputation, several studies assume that this molecule would cause allergies following its oxidation with air, and only on sensitive people. Linalool is used in the cosmetics industry for the production of essential oils and in the pharmaceutical industry for the manufacture of drugs.
Linalool is also considered one of the main contributors to the pleasant odor characteristics of strawberries. It may be one of the VOC substances that exert health benefits in these fruit (antimicrobial, neuroprotective, anti-inflammatory and antistress abilities). A study has. reported that linalool is a key component in strawberry VOC modulating gut microbiota, systemic inflammation, and glucolipid metabolism (Ton N. et al., Food Chem 10 July 2024, 140361).
Defensive role of simple terpenes have been demonstrated as for more complex compounds. Ocimene and linalool were shown to be produced by de novo biosynthesis in plants damaged by insect herbivories (Paré PW et al., Plant Physiol 1997, 114, 1161). These compounds likely mediate the interaction between herbivores and their natural enemies, attracted by terpenes. β-Ocimene is an example of chemical convergence between plants and insects, suggested to be important in pollinator attraction. Indeed, these aromatic molecules have the function of attracting pollinating insects, thus promoting the perpetuity of the species.This compound is also found in the genitals of male Heliconius butterflies. In Heliconius melpomene, (E)-β-ocimene acts as an anti-aphrodisiac pheromone, transferred from males to females during mating to repel further courtship from subsequent males. It is also found in large amounts in the flowers on which adult H. melpomene feed. This compound, therefore, appears to be carrying out 2 context-dependent functions, attraction to plants and repulsion from mated females (Darragh K et al., PlosOne 2021, 19: e3001022).
Experimentally, a study of Arabidopsis thaliana engineered to overexpress a terpene synthase leading to the emition of large amounts of linalool, which is normally produced only in trace levels. Compared with wild-type A. thaliana, the transgenic plants significantly repelled Myzus persicae aphids (Aharoni A et al., Plant Cell 2003, 15, 2866). That approach may be a solution to the protection of cultivated plants against insect attacks. Recent studies reveal a complex interplay between pollinator attraction and plant defense mediated by linalool and its derivatives. The diverse functions of linalool, ranging from toxin to long distance pollinator attractant are discussed in a review article (Raguso RA et al., Curr Op Plant Biol 2016, 32, 31).
Geraniol, produced by geranium, rose and lemon, has been determined to be also an alarm pheromone of the insect Corythucha ciliata which attacks the sycamore tree (Kuwahara Y et al., J Chem Ecol 2011, 37, 1211).
A great number of orchid species in the New World tropics have coevolved with the insects Euglossini by producing floral scents, highly attractive to insect males over great distances, for efficient pollination. Ipsdienol has been shown to be the main attractive component of the orchid scent (Schorkopf DL et al., J Chem Ecol 2011, 37, 953).
Ipsdienol
Citral which is a monoterpene aldehyde is a collective term for the E-isomer, named geranial (trans-citral) or citral A and the Z-isomer, named neral (cis-citral) or citral B. These natural stereoisomers occur mainly as a mixture. Citral is present in the essential oils of several plants. It is particularly present in high concentrations in oils of Backhousia citriodora (lemon myrtle) and in several species of Litsea, Ocimum and Lindera. It is also present in lemon and orange but at lower concentrations.
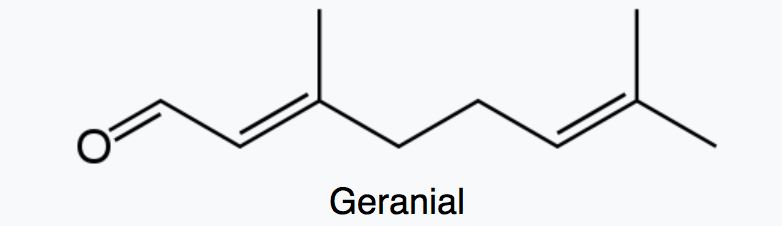
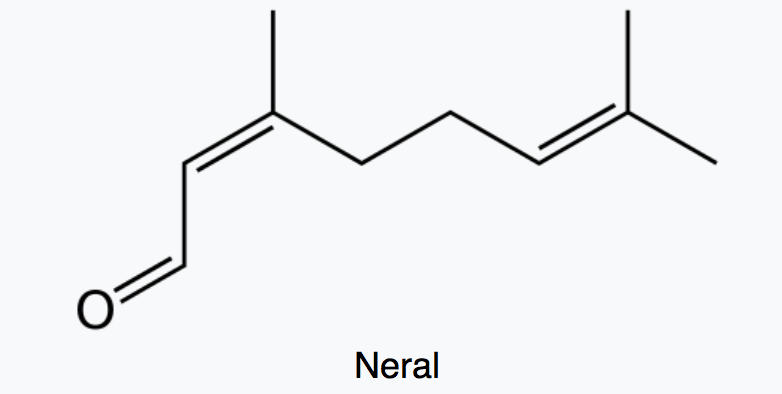
Citral has a strong lemon (citrus) scent and is used as an aroma compound in perfumery. It has also pheromonal effects in acari and insects. The herb Cymbopogon citratus rich in citral was shown to have promising insecticidal and antifungal activity against storage pests (Dubey NK et al., Curr Sci 1997, 73, 22).
A study has demonstrated that Litsea cubeba essential oil, which has a wide range of industrial uses, has also significant anti-tobacco mosaic virus activity (Jiang Y et al., Indust Crops Prod 2022, 183, 114948)
Citral was shown to be the active components of the oil. Experiments have also indicated that citral can induce plant resistance against fungal diseases.
Several halogenated monoterpenes (with Cl and/or Br atoms) have been isolated from a red alga Portieria hornemannii and showed significant anti-inflammatory activity to inhibit the production of pro-inflammatory cytokines in the LPS-stimulated mature dendritic cells (Wu YG et al., Mar Drugs 2023, 21, 493).
They are derived from cyclohexane with an isopropyl substituent. The most typical are :
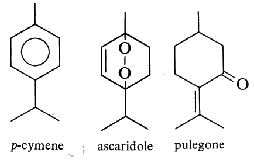
Limonene is one of the most common terpenoid in nature, it is an important volatile emitted by Pinus pinea (46% in volatiles), but also by the holm oak (Quercus ilex), it acts as allelochemical in inhibiting seed germination of other plant species (Singh HP et al., Ann Bot 2006, 98, 1261). About 30,000 tons/year of limonene are extracted from natural sources (turpentine oil) and are used for the syntheses of other optically active products (Schwab W et al., Eur J Lipid Sci Technol 2013, 115, 3). Studies have demonstrated that D-limonene, from citrus fruits, possesses antioxidant and cardiovascular properties. A study has demonstrated that d-limonene produces cardioprotective effects from isoproterenol-induced myocardial infarction through lowering of the amount of reactive oxygen species and suppression of apoptosis (Durco AO et al., J Nat Prod 2019, 82, 11, 3002). Several studies have reported that D‐limonene plays a valuable role in the prevention of several chronic and degenerative diseases. A review provides information about its beneficial effects such as antioxidant, antidiabetic, anticancer, anti‐inflammatory, cardioprotective, gastroprotective, hepatoprotective, immune modulatory, anti‐fibrotic, anti‐genotoxic etc.. (Anandakumar P et al., J Food Chem 2021, 45, e13566). Findings showed that limonene has the potential to be a natural nutrient regulator and play an important role in metabolic disorders induced by high fat diets (Wang QS et al., Food Biosci 2024, 105391). More recently, D-limonene has been found to have antidepressant-like properties, reducing anhedonic and defensive behaviours and the impairing effects of stress on learning and memory tests in rats (Alkanat M et al., Eur J Neurosci 2024, 60, 4491).
Carvone is found naturally in many essential oils, but is most abundant in the oils from seeds of caraway (Carum carvi), spearmint (Mentha spicata), mandarin orange peel oil and in several aromatic and medicinal plants of the Lamiaceae and Asteraceae families, including Thymus spp., Origanum spp., and many others. Chirality is important, R-(−)-carvone smells like spearmint while its mirror image, or enantiomer, S-(+)-carvone smells like caraway. Both carvones are used in the food and flavor industry. S-(+)-Carvone is used to prevent premature sprouting of potatoes during storage and R-(−)-Carvone is used as a mosquito repellent.
Pharmacological investigations showed that carvone exhibits multiple properties such as antibacterial, antifungal, antiparasitic, antineuraminidase, antioxidant, anti-inflammatory, and anticancer activities. A review has discussed the principal studies investigating the pharmacological properties of carvone, and the mechanism of action underlying some of these properties (Bouyahya A et al., Biomolecules
2021, 11, 1803). Investigations provided new insights into the antifungal and anti-aflatoxin B1 mechanisms of carvone, as well as providing theoretical support for the development of new fumigants to control Aspergillus flavus contamination.(Wei S et al., Food Biosci 2024, sept, 105117).
 Carvone
Carvone
Carvacrol, or cymophenol, is a monoterpenoid phenol. It has a characteristic pungent, warm odor of oregano (Origanum vulgare). It is present in the essential oil of oregano)but also in oil of thyme, pepperwort, and wild bergamot. In vitro, carvacrol has antimicrobial activity against different bacteria and fungi. The essential oil of thyme subspecies contains between 5% and 75% of carvacrol, that of oregano is also rich in carvacrol (50%).
 Carvacrol
Carvacrol
Carvacrol is an activator of the transient receptor potential cation channels V3 (TRPV3) and A1 (TRPA1). The pharmacological effects of pollen extracts have been attributed to carvacrol which inhibits NF-KB factors and thus reduces the level of prostaglandins E2 and increases the production of b-endorphins with inflammation decrease and pain rellief (Shaded AR et al., J Urol 2001, 166, 1738). A study has shown that treatment with carvacrol improved the functionality of endothelial progenitor cells, contributing to the reduction of endothelial dysfunction (Gonçalves TA et al., Nutrients 2023, 15, 3032). The effects of carvacrol on cardiovascular diseases has been reviewed (Mansi et al., Food Biosci, 2024, 57, 103444).
Thymol (2-isopropyl-5-methylphenol) is a derivative of p-cymene, isomeric with carvacrol, found in oil of thyme (Thymus vulgaris), it has a pleasant aromatic odor and strong antiseptic property. It has gained attention for its diverse biological properties, including antioxidant, anti-inflammatory, and immunomodulatory activities. Thymol supplementation could provide potential neuroprotection and improve cognitive deficits, depressant-like effects, learning, and memory impairments (Peng X et al., J Agric Food Chem 2024, 72, 6803).
 Thymol
Thymol
Terpineol has been isolated from a variety of sources such as cardamom, cajuput oil, pine oil. Four isomers exist: α-, β-, γ-terpineol, and terpinen-4-ol. Terpineol is usually a mixture of these isomers with α-terpineol as the major constituent. It has a pleasant odor similar to lilac and is a common ingredient in perfumes, cosmetics, and flavors.
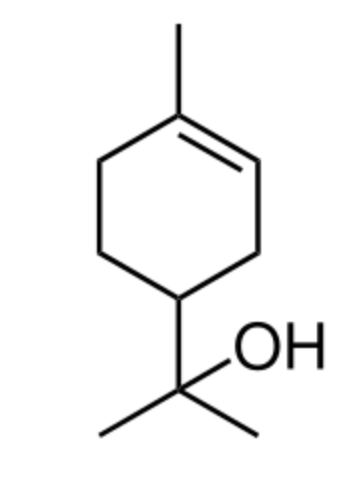
Terpineol
α-Terpinyl acetate, a terpineol acetate ester, is a commercially important fragrance molecule. It offers a smooth and pleasant aroma reminiscent of a blend of herbs, lavender, and bergamot. The essential oils obtained from Stachys setifera, Chamaecyparis, and Thymus willkomii contain α-terpinyl acetate as one of their main components. Investigations have shown that it demonstrated anti-cholinesterase, anti-oxidative, anti-amyloidogenic and neuroprotective potential and thus could be used as a lead to develop a novel, safe and effective therapy for Altzeimer’s disease (Chowdhury S et al., J Functional Foods 2020, 68, 103892). It has been demonstrated that α-terpinyl acetate was an efficient inhibitor of cytochrome P450 2B6 enzymatic activity (Lee Y et al., Chem Biolo Inter 2018, 289, 90). These results may provide important insights into the future development of therapeutics and the prediction of their possible adverse side effects.
Hinokitiol (or β-thujaplicin) is a compound found in the wood of trees in the family Cupressaceae (first extracted from Chamaecyparis taiwanensis). It is a tropolone derivative which was the first non-benzenoid aromatic compound identified.
used in oral and skin care products and is a food additive used in Japan. It has also shown to have insecticidal, pesticidal and antibrowning effects. These properties could be due to its metal ion-binding activity (iron-chelating activity) (Wikipedia).
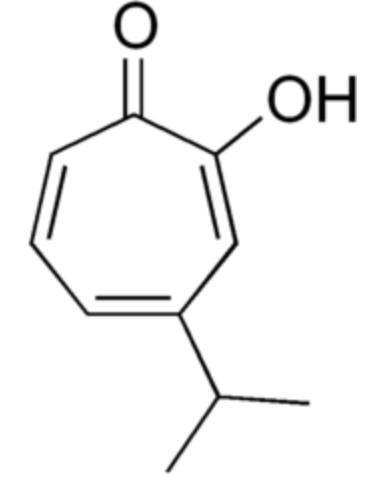 Hinokitiol
Hinokitiol
Hinokitiol also exhibits several pharmacological properties, including antidiabetic, anti-inflammatory, anticancer and antimicrobial effects (El Hachlaf N et al., Processes 2021, 9(9), 1680).
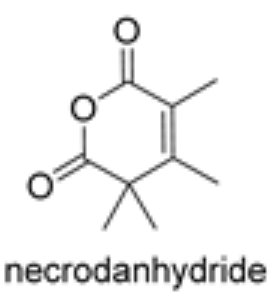
Necrodanhydride is a monoterpenic anhydride isolated from Lavandula luisieri and other plants living in the Iberian Peninsula. This compound is only present in the aerial parts. It was proved to be a potent nematicide against Meloydogine javanica, phytotoxic in tests on Lactuca sativa and Lolium perenne and ixodicid against Hyalomma lusitanica (Molina Inzunza DO et al., Biomolecules 2024, 14(8), 955).
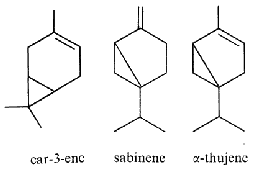
Thuyone is best known for being a toxic chemical (the a form is the most active) in absinthe, a product extract from Artemisia absinthium. Its psychedelic effects consecutive to absinthe consumption is disputed. Pharmacologically, thujone acts mainly on the GABA receptors in the brain and exhibits psychoactive response. In many countries the amount of thujone allowed in food or drink products is regulated (in Europe, the maximum level tolerated is 25 mg/l). Other plants containing thujone, such as the coniferous Thuja occidentalis, are used in herbal medicine, mainly for their immune-system stimulating effects. The use of thujone and thujone-containing plant parts for human consumption is currently regulated by the European Parliament and Council and the European Medicines Agency. A review describes the main steps of the biosynthetic route of thujones, their biological activities and their occurrence in the plant kingdom (Németh EZ et al., Phytochem Rev 2020, 19, 405).
Pinene (α- and β-isomers) is the major component of the volatiles emitted by Pinus pinaster (75% of the total). Pinene, as limonene, is an allelochemichal emitted by the roots of Quercus ilex. Pinene is among the more readily available optically active substance (about 30,000 tons/year) and is used for the syntheses of other chemical products (Schwab W et al., Eur J Lipid Sci Technol 2013, 115, 3).
Camphor and pinene are also allelochemicals emitted by Salvia leucophylla (Nishida N et al., J Chem Ecol 2005, 31, 1187).
Camphene is also a bicyclic terpene. It is one of the most pervasive monoterpenes. it is insoluble in water, flammable, colorless, and has a pungent smell. It is a minor constituent of many essential oils such as turpentine, cypress oil, camphor oil, citronella oil, neroli, ginger oil, valerian, and mango. Camphene has been shown to have anti-inflammatory and hypolipidemic properties. It may also protect the heart against ischemia/reperfusion injury by maintaining redox homeostasis (Stamatiou R et al., Antioxidants 2024, 13, 405).
 Camphene
Camphene
Verbenol (2-pine-4-ol) is a group of four stereoisomeric monoterpene alcohols derived from pinene. They have been found to be active components of insect pheromones and essential oils. Thus, trans-Verbenol is a mountain pine beetle (Dendroctonus ponderosae) pheromone that attracts insects to a tree. Reservoirs of trans-verbenol has been described in the bodies of juvenile mountain pine beetles living in western North America (Chiu CC et al., PNAS 2018, 115, 3652). Furthermore, the female adult uses that compound to attract other insects to a suitable host tree and coordinate large-scale attacks.
(-)-trans-Verbenol
3-Carene consists of fused cyclohexene and cyclopropane rings. It occurs as a constituent of turpentine, with a content as high as 42% depending on the source. Carene has a sweet and pungent odor, best described as fir needles, musky earth, and damp woodlands combination. It is present in mango with a pine-like flavor and aroma, explaining its use in the perfume industry. Several studies have reported that carene has anti-asthmatic and anti-inflammatory properties (Kim K et al., J Physiol Pharmacol 2024, 75, 195). Carene has also been used to synthesized new compounds exerting good herbicidal activities (Cui Y et al., J Agric Food Chem June 10, 2025).
Fenchol (1,3,3-trimethyl-2-norbornanol) is an isomer of borneol. It occurs widely in nature. The naturally occurring enantiopure (1R)-endo-(+)-fenchol gives basil its characteristic scent and is also used extensively in perfumery.
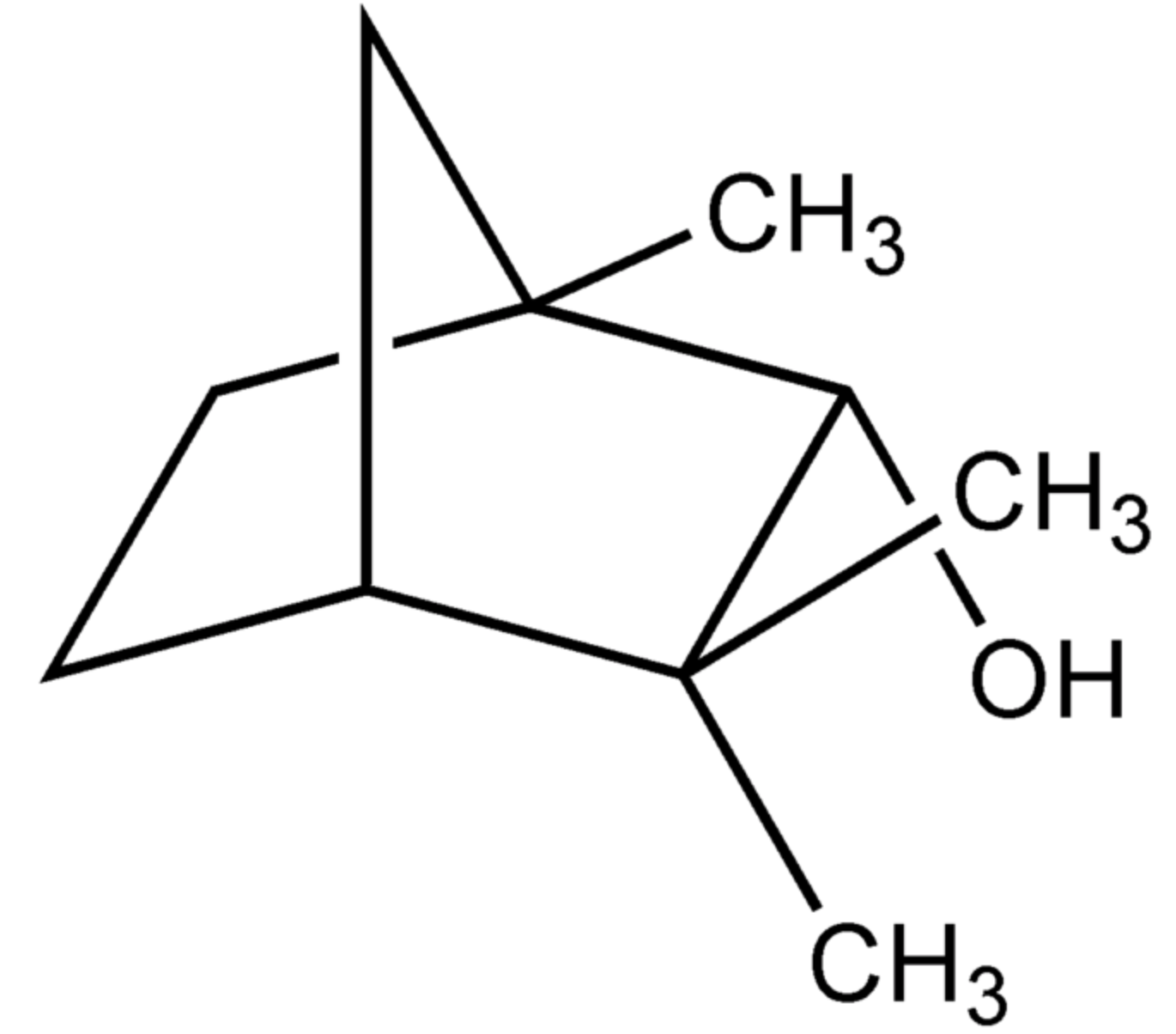
Fenchol
Fenchol was recognized as a potential stimulator of the FFAR2 receptor in neuronal cells and it demonstrated protective effects against Aβ-stimulated neurodegeneration (Razazan A et al., Front Aging Neurosci 2021, 13:735933). Thus, stimulation of FFAR2 signaling by fenchol as a natural compound can be a therapeutic approach to ameliorate the pathology of Alzheimer’s disease.
2-Methylisoborneol is with geosmin accounts for the majority of biologically-caused taste and odor outbreaks in drinking water worldwide. It has a distinct earthy or musty odor, which most people can easily smell. It is also a factor in cork taint in winemaking (Wikipedia).
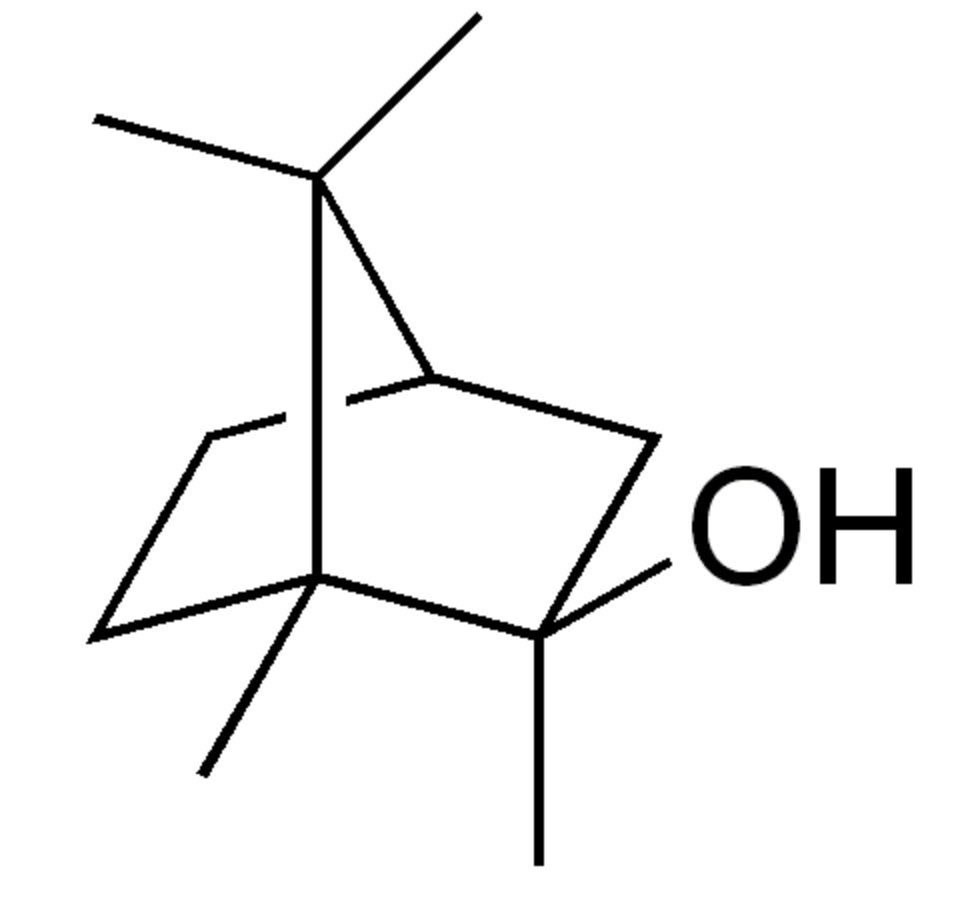 2-Methylisoborneol
2-Methylisoborneol
Eucalyptol (also called 1,8-cineole) is a bicyclic ether. It has a camphor-like odor and a spicy taste. It makes up about 50–90% of eucalyptus oil and camphor oil, and 20-60% of cardamon oil (Hamdy SA et al., Food Chem 26 August 2024, 141009). It was identified in 1870 by Cloez F in Eucalyptus globulus oil and named “eucalyptol”. It is used in flavorings, fragrances, and cosmetics. Eucalyptol is also essential in pharmaceutical products thanks to its antifungal, anti-infectious, bactericidal, antiviral and expectorant properties. Eucalyptol is commonly used in the treatment of respiratory conditions (bronchitis, colds), asthma attacks and sore throats. A review sets the stage for future research on diverse health benefits and potential uses of 1,8-cineole in tackling complex medical conditions (Hoch CC et al., Biomed Pharmacother 2023, 167, 115467).
It has in general insect repellent properties but is attractive to males of various species of orchid bees (Schiestl FP et al., J Chem Ecol 2004, 29, 253).
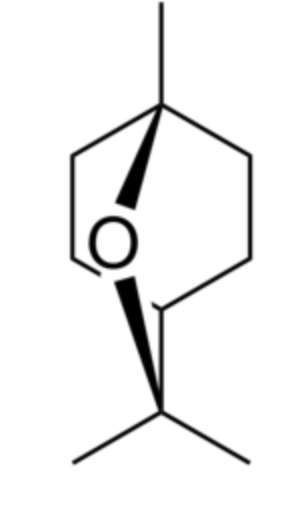 Eucalyptol
Eucalyptol
Iridoids are a class of bicyclic monoterpenes found in a wide variety of plants and in some animals. They are often intermediates in the biosynthesis of alkaloids. They protect these plants from herbivorous insects by functioning as insect repellents but are also sex pheromones in many aphids.
Chemically, iridoids usually consist of a cyclopentane ring fused to a six-membered oxygen heterocycle, as exemplified by nepetalactone, the active ingredient in catnip (Nepeta cataria), plant known for being cat attractants, however in only about two thirds of adult cats. They produce similar behavioral effects in many other felids, especially in lions and jaguars but not tigers.
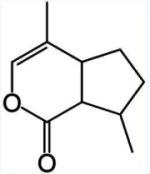
Nepetalactone
Actinidine is an other iridoid produced in nature by a wide variety of plants and animals having also the property of cat attractant. It was the first cyclopentanoid monoterpene alkaloid to be discovered (Wikipedia).
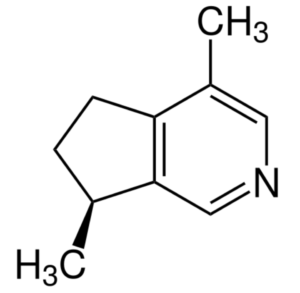 Actinidine
Actinidine
Iridoids are typically found in plants as glycosides, most often bound to glucose.
Cleavage of a bond in the cyclopentane ring gives rise to a subclass known as seco-iridoids.
Iridoids are found in many medicinal plants and may be responsible for the some of their pharmaceutical activities. Isolated and purified, iridoids exhibit a wide range of bioactivities including cardiovascular, hypoglycemic, anti-inflammatory, antispasmodic, antitumor, antiviral, immunomodulator and purgative activities (Didna B et al., Chem Pharm Bull 2007, 55, 159). They are produced by plants primarily as a defense against herbivores or against infection by microorganisms. To humans and other mammals, they have also a deterrent bitter taste. It can also be used as a mosquito repellent. As other terpenes, iridoids may function as protective substances in the animal kingdom, especially for insects. These compounds are obtained from the diet or, as the iridoids of leaf beetles, they are made by the insect itself.
Many monoterpenes possess antitumor activity in animal and cell models. They have also antioxidant properties, γ-terpene and hydroxytyrosol being among the most effective.
Devenez membre et participez au développement de la Lipidomique au XXIème siècle.
S'inscrire