
Faraday Michael determined in 1829 that rubber produced by Hevea brasiliensis was made up solely of carbon and hydrogen and had the empirical formula C5H8. In 1860, the English chemist Greville Williams C obtained a liquid with the same formula by distilling rubber, he called it isoprene (Williams CG, Philos Trans Roy Soc London 1860, 150, 241). In 1879, Bouchardat G obtained isoprene from natural rubber and found that heating isoprene with HCl produced a rubber-like polymer after distillation. He said that this new product had “the elasticity and other properties of rubber itself”. This was the first production of artificial rubber. After the early demonstration that each isoprene unit has one double bond and that rubber has a high molecular weight, the idea that the rubber molecule consisted of long chains formed by the regular linking of isoprene units was only slowly established after the works of Harries CD (between 1902 and 1905) and mainly of Staudinger H (in 1920) who coined the term “macromolecule”. It was determined that the hydrocarbon chains were composed of an initial group w‘ formed by two or three trans-isomer units, a long chain formed by a great number of cis-isomer units, and a not yet completely determined terminal group a (Tanaka Y et al., Kautschuc Gummi Kunststoffe 1997, 50, 6).
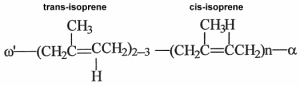
The w-terminal linking to proteins was suggested to form physical cross-links, whereas the a-terminal linking to phospholipids to form chemical cross-links with long chain fatty acid ester groups. It was shown that the linked fatty acids were composed of saturated and unsaturated C10 to C22 fatty acids, the composition of which was similar to that of mixed fatty acids (Kawahara S et al., Polymer 2000, 41, 7483). It seems that the mechanical properties of natural rubber could be dependent on the composition of these fatty acids
If natural rubber is formed by cis-isomer units, the material known as gutta-percha produced by Palaquium gutta (Sapotaceae), and balata, formed by Mimusops globosa, are polyisoprenes having all a trans structure.
The polymer chains of rubber are very long and have an average molecular weight more than a million. As these long chains are not naturally cross-linked, rubber is soluble in non-polar solvents and thus may be considered as lipids.
Goodyear C found in 1830 the way to harden the natural rubber in heating the raw product with elementary sulfur, process which creates chain cross-links and is now known as vulcanization.
It must be noticed that while natural rubber is mainly produced today from Hevea tree, it may be also obtained from guayule (Parthenium argentatum), a xerophytic shrub that has been exploited as commercial sources rubber since the pre-Columbian times when Indians of Mexico used it to form balls for their games.
DISPERSIVE LIQUID-LIQUID MICROEXTRACTION
Lire la suiteDevenez membre et participez au développement de la Lipidomique au XXIème siècle.
S'inscrire