
These lipids (known also as triglycerides) are fatty acid triesters of glycerol and may be divided into three general types with respect to their acyl substituents. They are simple or monoacid if they contain only one type of fatty acid, diacid if they contain two types of fatty acids and triacid if three different acyl groups. In some triacylglycerols, fatty acids are replaced by phenolic acids.
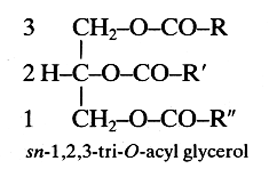
R, R’ and R” are saturated or unsaturated chains.
From his research on saponification, Chevreul suggested in his famous work (Recherches chimiques sur les corps gras d’origine animale, Paris 1823) that fats, as neutral bodies, could be considered similar to the ethers of the third class (esters) of the Baron Thénard (1777-1857), professor at the Collège de France. Thus, he described fats as combinations of fatty acids with a substance which adds water under the influence of alkali to form glycerin, this substance being analogous to alcohol. Studies by H. Braconnot on animals fats, released in 1815, led to preliminary conclusions on the way of the true nature of triglycerides. It must be recalled that about 150 years before these investigations, a German chemist, Otto Tachenius, has suggested that oils and fats contain a “hidden acid” which forms a salt (soap) when reacting with alkali.
After these first hypotheses, the problem of the constitution of fats (triglycerides) remained unresolved until the first successful synthesis in 1844 of a triglyceride molecule (tributyrin) by T.J. Pelouze, in reacting butyric acid with glycerin in the presence of concentrated sulfuric acid (Ann Chim Phys 1844, 10, 434) and the synthesis of tristearin and tripalmitin by one of his student, Berthelot M, in 1853-1854 (C R Séances Acad Sci, Paris, 1853, 36, 27; Ann Chim Phys 1854, 41, 216) in reacting at 100°C fatty acids and glycerin in the presence of gaseous HCl.
From the original work of Chevreul it was thought up to the half of this century that natural fats were mixtures of simple triacylglycerols. With new technologies, Hilditch devised new analysis which revealed that all natural fats were composed largely of mixed triacylglycerols (Hilditch TP et al, The chemical constitution of natural fats, Chapman & Hall, London, 1964). Later confirmation was given that natural fats and oil are formed of complex mixtures of various molecular species. This complexity can be deduced from the fatty acid analyses of purified triacylglycerols from vegetal or animal origin. Milk fat is an example of complex mixtures since 223 individual molecular species, accounting for about 80% of total triacylglycerols, were determined in 1993 using several complementary techniques (Gresti J et al, J Dairy Sci 1993, 76, 1850).
The composition and structure of natural vegetal oils may be now modified by chemical or enzymatic processes known as interesterification.
An extensive review of the biosynthesis of triacylglycerols has been released by Lehner R and Kuksis A (Prog Lipid Res 1996, 35, 169).
The life cycle of triacylglycerols, their synthesis, storage and degradation, has been reviewed (Athenstaedt K et al., Cell Mol Life Sci 2006, 63, 1355).
Triglyceride configuration analysis reveals that fatty acid positioning exhibits source-dependent variability, with sn-2 positional isomerism critically determining enterocytic absorption kinetics. Thus, marine-derived triglycerides have preferentially DHA at the sn-2 site, whereas plant-sourced lipids have predominantly omega-3 fatty acids at sn-1/3 positions. Pancreatic lipase selectively cleaves terminal ester bonds during hydrolysis, thus preserving sn-2 fatty acids (as 2-monoacylglycerols). The resultant sn-2-bound fatty acids undergoes efficient enterocyte uptake via passive diffusion and membrane transporter-mediated processes, while free fatty acids from sn-1/3 positions require re-esterification. Mechanistic studies have proposed that preserved sn-2 monoacylglycerol structure facilitates lipoprotein receptor-mediated transcytosis at the brush-brain membrane interface improving spatial memory, cognitive performance, and reducing oxidative stress and serum lipids (Zhong Y et al., Food Bioscience June 2025, 106998).
Some acylglycerols have been proven to be suitably meltable excipients able to sustain drug release from pharmaceutical oral dosage forms (Jannin V. et al., Adv Drug Deliv Res 2008, 60, 734-46). In this field, triacyl behenate (22 carbon-chain) excipient presents special interest due to its pronounced hydrophobic and its temperature around 70°C. Compritol® 888 is the most known component to trap drugs in a solid phase formed upon cooling.
Alkyl-diacylglycerols
These compounds, analogs of triacylglycerols, are found in liver oils from marine organisms, particularly elasmobranchs (10-30 % in liver oil) but are also found in mammalian tumor lipids. They are also considered as a major storage lipid in the zooplankton pteropod, genus Clione, from polar and temperate regions. Up to 41% of the lipid of the pteropod C. limacina in polar oceans can be diacylglycerol ethers (Kattner G et al., Mar Chem 1998, 61, 219).
Saponification cleaves the two esters bonds and frees monoalkyl glycerols. They were discovered in 1922 by Tsujimoto and Toyama.
Alkyl-diacylglycerols are present in bone marrow (0.2 %), mammalian milks (0.1 % in human milk, 0.01% in cow milk) and colostrum (Hallgren B et al., Acta Chem Scand B 1974, 28, 1029) and are said to have antioxidant action, to inhibit cancer cell growth, to prevent damage from radiation therapy, and to boost the immune system. An alkyl-diacylglycerol species with two acylated oleic acids in position 2 and 3 and an alkylated stearic chain in position 1 has been detected in honey of various botanical origins (Schievano E et al., J Agric Food chem 2013, 61, 1747).
Coral total lipids contain a significant quantity of monoalkyldiacylglycerols (Imbs AB et al., Mar. Drugs 2023, 539). In corals that house zooxanthellae, these lipids are found only in the total lipids of the pure polyp tissue fraction, suggesting that they could serve as a lipid marker for the host in the symbiotic relationship of the coral. It was shown that the concentration of monoalkyldiacylglycerols in the total lipids varied between 1.0 and 9.5%, the highest concentration being observed in the soft coral. The gorgonian coral Paracis exhibited the highest monoalkyldiacylglycerol concentration at 51.9% of its total lipids.
The primary alkylglycerols derived from these lipids were shown to be chimyl alcohol (1-hexadecylglycerol) and batyl alcohol (1-octadecylglycerol) with trace amounts of selachyl alcohol (1-octadeca-9-enylglycerol).
![]()
From a world production of about 20 million tons in 1939, about 77 million tons were produced in 1989 whose 74% were of vegetal origin. In 1999-2000 the global production of oils and fats was about 113 million tons, in 2004-2005 it was about 136 million tons (with 82% of vegetal origin). In 2011/12, the production of vegetable oils was about 157 million tons. The annual world average consumption of oils and fats in 2003 is about 20 Kg per capita. Most of oils and fats is used for food purposes (80%), or in oleochemistry (14%), or is applied as animal feed (6%).
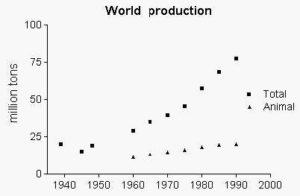
In the European community about 8 million tons of fats and oils will be used for human consumption in 2029 (source: Statista).
Since the mid 1970s, fuel shortages spurred interest in diversifying fuel sources in Europe and later in some countries, and biodiesel was produced from various vegetal oils as an alternative to petroleum diesel.
Of the total amount of fats and oils which is produced worldwide, by far the largest share was used in human foodstuffs. For oleochemistry, about 15 million tons are available. In recent years, the amounts produced have continuously increased by approximately 3% per year. It is predicted that this trend will continue in the medium and long terms. Unexpectedly, it may be recalled that about 550 000 and 300 000 tons of candles, made mainly of saturated vegetal oils, were used in USA and in European countries, respectively.
Many claims concerning the potential of novel oil-based products from genetically engineered crops were announced. Scientific advances suggest the possibility to produce novel fatty acids with chain lengths from C8 to C24 and with a wide range of industrially useful functionalities. The promising market sectors and product ranges for the future development of these new oil crop biotechnology have been discussed by Murphy DJ (Phytochemistry Rev 2002, 1, 67).
![]()
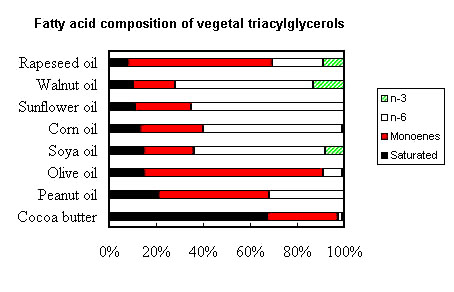
To know the sources and the composition of
fats and oils,
click below:
Devenez membre et participez au développement de la Lipidomique au XXIème siècle.
S'inscrire