
Cholesterol has been exploited with great advantage to detect any oxidation process in cell membranes. In contrast with unsaturated fatty acids, cholesterol exists as a single molecular species, its oxidation products are thus much less complicated to isolate and characterize (Smith LL, Cholesterol autoxidation, Plenum Press, NY 1981).
Cholesterol may undergo autoxidation and photo-oxidation, both processes give rise to oxysterols of various structures depending on the type of oxidation and the physical state of the substrate. Thus, the identification of cholesterol oxidation products may be used as a mechanistic proof in various oxidant systems. When cholesterol esters are oxidized, the structure and the yield of the formed oxysterols depend on the fatty acid species.
An extensive review on oxysterols, including their analysis, formation, occurrence, metabolism and physiological actions, may be consulted for further information (Schroepfer GJ, Physiol Rev 2000, 80, 362). The physiological effects of oxysterols has been extensively reviewed (Mutemberezi V et al., Prog Lipid Res 2016, 64, 152).
Phytosterols may undergo oxidative processes comparable to those involved in cholesterol oxidation but the amount of biological research on oxyphytosterols is yet scarce. A review summarizing the present knowledge of biological effects of oxidized phytosterols may be consulted (Hovenkamp E et al., Prog Lipid Res 2008, 47, 37).
![]()
1 – AUTOXIDATION PRODUCTS
– Cholesterol molecule
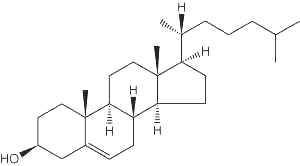
Cholesterol
The most important oxysterols are generated intracellularly by cholesterol hydrolases belonging to the cytochrome P450 complex. Of these by-products, the most important in humans are 27-, 24-, 7α-, and 4β-hydroxycholesterol. They may also arise in vivo or during food processing through several chemical processes, termed cholesterol autoxidation. The most important autoxidation products are 7-keto and 7β-OH cholesterol.
In simplifying the very complicated situation, it can be considered that the reaction of cholesterol with free radicals gives rise mainly to:
– C-7 oxygenated molecules
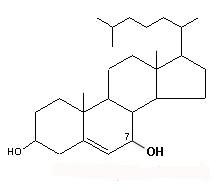
7α-OH or 7β-OH cholesterol
These molecules are further transformed into hydroxylated derivatives or into:
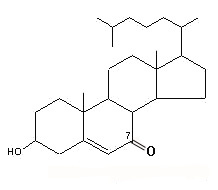
7-keto cholesterol
Findings have suggested that 7-keto cholesterol is at least partially responsible for cholesterol-induced bone loss by inducing autophagy in osteoclasts (Sul OJ et al., J Nutr Biochem 2021, 96, 108783).
– C-5 + C-6 oxygenated derivatives, i.e., 5,6α– or 5,6β-epoxides
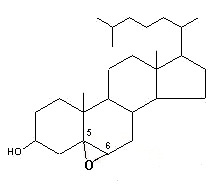
These molecules are further transformed into a triol derivative:
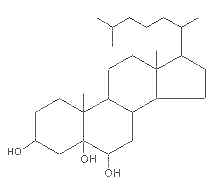
It is well known that these compounds display cytotoxic, pro-apoptotic, and pro-inflammatory activities. Several investigations suggest that they act as endogenous regulators of gene expression in lipid metabolism and as cell signaling molecules (review in : Olkkonen VM, Lipids Insights 2008, 2, 1).
A review of all the aspects of the sources, cellular storage and metabolism of the oxysterols as been released by Brown AJ et al. (Mol Aspects Med 2009, 30, 111). The implications in the pathology of degenerative diseases have been reviewed in 2009 (Lordon S et al., J Nutr Biochem 2009, 20, 321). Numerous studies show that oxysterols are associate with various types of cancer. A review of the molecular mechanisms which contribute to the initiation and progression of cancers may be consulted (Jusakul A et al., Lipids Health Dis 2011, 10, 44).
The analysis of oxysterols is most efficiently done using modern techniques such as liquid chromatography coupled with mass spectrometry. A highly sensitive procedure based upon a stable isotope dilution technique byliquid chromatography with tandem mass spectrometry has been described (Honda A et al., J Lipid Res 2009, 50, 350).
2 – PHOTO-OXIDATION PRODUCTS
Peroxidation of cholesterol by singlet oxygen produces primarily a C-5 oxygenated molecule
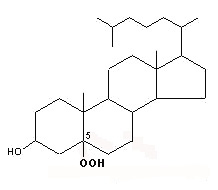
5 α-hydroperoxycholesterol
This molecule may be later rearranged giving 7α-hydroperoxycholesterol which is progressively epimerized into 7β-hydroperoxycholesterol.
3 – ENZYMATIC OXIDATION
Further complications arise from the possible in vivo formation of some other oxysterols by enzymatic reaction.
If these reactions form mainly oxygenated derivatives in C-7 position, other products result from reactions on the lateral chain at C-20, C-22, C-24, C-25 and C-27.
24S-Hydroxycholesterol (cerebrosterol) is an enzymatically oxidized product of cholesterol mainly synthesized in the brain (Ercoli A et al., J Am Chem Soc 1953, 75, 3284). An overview of several studies on cerebrosterol has been presented with a discussion about its possible connection with neurodegenerative diseases (Bjorkhem I, Lipids 2007, 42, 5). It must be noticed that 24S-Hydroxycholesterol was found in high concentration in atheroma plates.
The oxysterol 24(S),25-epoxycholesterol is made in a shunt in the cholesterol biosynthetic pathway in all cholesterogenic cells.
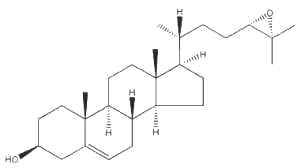
24(S),25-epoxycholesterol
Evidence is emerging that it can work at several levels to control acute cholesterol homeostasis. For instance, this oxysterol repressed the activity of a key rate-controlling enzyme in cholesterol biosynthesis, hydroxymethylglutaryl (HMG)-CoA reductase. In addition, endogenous 24(S),25-epoxycholesterol is a natural ligand for the liver X receptors which induce expression of cholesterol efflux-related genes (Brown AJ, Int J Biochem Cell Biol 2009, 41, 744).
It has been demonstrated that 24,25-epoxycholesterol promotes midbrain dopaminergic neuron neurogenesis (Theofilopoulos S. et al., J Biol Chem 2019, 294, 4169). The authors propose that increasing the levels of 24,25-epoxycholesterol in vivo may be a useful strategy to combat the loss of dopaminergic neurons in Parkinson’s disease.
DISPERSIVE LIQUID-LIQUID MICROEXTRACTION
Lire la suiteDevenez membre et participez au développement de la Lipidomique au XXIème siècle.
S'inscrire