
Fatty acids may be naturally derivatized with a methoxy or an acetoxy group.
Naturally occurring a-methoxy fatty acids were identified in phospholipids from various sponge species living in warm waters : saturated, monounsaturated and diunsaturated 2-methoxylated fatty acids (19 to 24 carbon atoms) were described (Ayanoglu E et al., Lipids 1983, 18, 830; Carballeira NM et al., Lipids 1992, 27, 72; Carballeira NM et al., J Nat Prod 1998, 61, 675; Carballeira NM et al., Lipids 2007, 42, 1047).
Among them, 2-methoxy-5-hexadecenoic acid, 2-methoxy-6-hexadecenoic acid and 2-methoxy hexadecanoic acid were identified in phospholipids of Caribbean sponges.
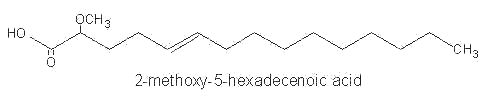
These monounsaturated fatty acids were shown to have antimicrobial activity.
All these compounds were postulated to originate from bacteria in symbiosis with sponges.
Several other saturated and unsaturated 2-methoxylated fatty acids were isolated from phospholipids of a Caribbean sponge (Callyspongia fallax) (Carballeira NM et al., J Nat Prod 2001, 64, 620). The saturated were identified as 2-methoxytetra-, penta- and octadecanoic acids, the monounsaturated as 2-methoxy-6-tetra-, penta- and hexadecenoic acids.
The 2-methoxy-13-methyltetradecanoic acid was identified, together with other 2-methoxylated C15-C16 fatty acids, in the sponge Amphimedon complanata from Puerto Rico (Carballeira NM et al., Lipids 2001, 36, 83). This fatty acid is the methoxylated analog of the bacterial 2-hydroxy-13-methyltetradecanoic acid and was shown to be highly cytotoxic to human cancerous cells (Carballeira NM et al., Chem Phys Lipids 2003, 126, 149). These methoxylated fatty acids could have originated from bacteria in symbiosis with the sponge.
Two 2-methoxy fatty acids with a very long carbon chain (2R,5Z,9Z)-2-methoxy-25-methyl-5,9-hexacosadienoic acid and (2R,5Z,9Z)-2-methoxy-24-methyl-5,9-hexacosadienoic acid were isolated from the Caribbean sponge Asteropus niger and were shown to be effective topoisomerase IB inhibitors (Carballeira NM et al., Lipids 2016, 51, 245–256).
Several saturated 2-methoxylated fatty acids (from C10 to C14) were synthesized and were shown to display some degree of inhibition of Mycobacterium tuberculosis (Carballeira NM et al., Lipids 2004, 39, 675).
Fatty acids methoxylated at other positions have been identified from a few natural sources. The marine cyanobacterium Lyngbya majuscula contain 7-methoxy-4-tetradecenoic acid (Cardellina JH et al., Phytochemistry 1978, 17, 2091), 9-methoxypentadecanoic acid and 15-methoxytricosanoic acid were identified in the red algae Schizymenia dubyi (Barnathan G et al., Phytochemistry 1998, 47, 761). Other acids have originated from bacteria, such as 11-methoxyheptadecanoic and 11-methoxynonadecanoic acids identified in Helicobacter pylori from human gastric mucosae (Inamoto Y et al., J Gastroenterol 1995, 30, 315). 10-methoxyoctadecanoic, 11-methoxyeicosanoic and 13-methoxynonadecanoic acid were detected in the bacterium Thiobacillus (Kerger BD et al., FEMS Microbiol Lett 1986, 38, 67).
Unusual long-chain methoxylated fatty acids were detected in the fungi Blumeria graminis (the causal agent of wheat powdery mildew) (Muchembled J et al., Phytochemistry 2005, 66, 793). They were identified as 3-methoxydocosanoic and 3-methoxytetracosanoic acids.
The phospholipids of the sponge Polymastia gleneni were shown to contain saturated long chain (C-22 to C-30)-acetoxy fatty acids (Ayanoglu E et al., Lipids 1985, 20, 141).
In plants, several 3-acetoxy fatty acids with 14, 16 or 18 carbon atoms are constituents of the partially acetylated acylglycerols found in floral oils of several species of Diascia (Srophulariaceae) (Dumri K et al., Phytochemistry 2008, 69, 1372). In Calceolaria and Lysimachia, a diacylglycerol, (1,2-di-(3-acetoxy-11-octadecenoy)-sn-glycerol) is the major floral lipid.
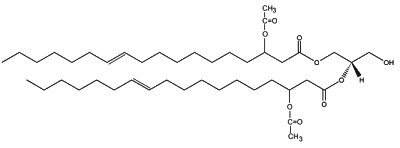
1,2-di-(3-acetoxy-E-11-octadecenoy)-sn-glycerol
From Byrsonima intermedia (Malpighiaceae) floral oil, a di-acetylated fatty acid was isolated, 3,7-diacetoxy-docosanoic acid (bryonic acid) (Reis MG et al., J Chem Ecol 2007, 33, 1421). Several partially acetylated dihydroxy fatty acids could be identified in the floral oil secreted by Malpighia coccigera (Malpighiaceae) (Seipold L et al., Chem Biodiv 2004, 1, 1519). These fatty acids had a chain of 20, 22 or 24 carbon atoms and two hydroxyl groups in various positions, one of them being acetoxylated.
Unexpectedly, these compounds characterize non-volatile oils produced in flowers by special anatomical adaptations (elaiophores) which are collected by pollinating bees, mainly in tropical areas of North and South America (neotropic ecozone). The bees seem to use these lipid secretions (instead of nectar) as provision for their larvae. These structures, generating a “floral syndrome”, were described for the first time by Vogel S in 1969 (XI Proc Intl Bot Congress, Seattle, 1969, p. 229. Abstr). In 1987, a review on the ecology of oil flowers and their bees (more than 400 species) listed more than 2400 species of flowering plants (10 families) offering fatty oils (Buchmann SL, Ann Rev Ecol Syst 1987, 18, 343).
A group of unusual triglycerides, in which one of the acyl groups is a vicinal dihydroxy acid with one of the hydroxyl groups acetylated, has been isolated from Cardamine impatiens (Cruciferae) seed oil. Several species were identified : C-18, C-20, C-22, and C-24 hydroxy acetoxy fatty acids (Mikolajczak KL et al., Lipids 1968, 3, 215).
DISPERSIVE LIQUID-LIQUID MICROEXTRACTION
Lire la suiteDevenez membre et participez au développement de la Lipidomique au XXIème siècle.
S'inscrire