
1 – Branched chain fatty acids are found most frequently with an unsubstituted carbon chain (some branched polyunsaturated fatty acids are found in sponges) but may have one or several branched methyl groups :
Mono or multibranched chain fatty acids
2 – Branched chain fatty acids (mono- branched) may have also a methoxy or a hydroxy substitution, they are found in exotic animals or bacteria :
Branched methoxy fatty acids
Branched hydroxy fatty acids
(Mycolic acids)
![]()
As for hydrocarbons, they have usually either an iso-structure (methyl group at the penultimate carbon atom) or a anteiso-structure (methyl group on the third carbon from the end).
Examples: 14-methyl pentadecanoic acid (isopalmitic) is of the iso-series and 13-methyl pentadecanoic acid is the corresponding anteiso-acid.
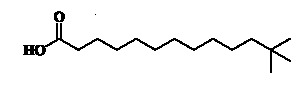
1 – Monomethyl branched fatty acids
They are found in vegetal, animal, and microbial lipids but in small concentrations. In animals, some classic examples of these compounds include the 2- and 4-monomethylated fatty acids from the uropygial gland of ducks (Kolattukudy PE et al., Arch Biochem Biophys 1991, 284, 201), as well as from the guinea pig Harderian gland (Yasugi E et al., J Biochem 1991, 110, 202). Iso– and anteiso-fatty acids from 8 to 30 carbons are present in high proportions in sebum (Nordstrom KM et al., J Invest Dermatol 1986, 86, 700) and in vernix caseosa (biofilm covering the human fetus) (Rissmann R et al., J Invest Dermatol 2006, 126, 1823).
18-Methyl-eicosanoic acid (anteiso form) is the most abundant fatty acid in the hair of most mammals, including humans, It makes up as much as half (38-47 %) of all the fatty acids in hair and is found exclusively on the surface of the cuticle (Wurtz PW et al., Comp Biochem Physiol B, 1989, 92, 759). It is covalently linked to the exterior surface of the hair protein via thioester bonds where it functions to maintain a waterproof barrier to the hair surface. It can be extracted only after the hair has been subjected to alkaline hydrolysis.
In vegetals, 14-methyl-16:0 has been identified in Ginkgo biloba (Hierro MTG et al., J. Am Oil Chem Soc, 1996, 73, 575) and was found to be characteristic of pine seed oil (up to 1%). This fatty acid was found exclusively in Pinaceae (genera Pinus, Abies, Cedrus, Picea …) (Wolff RL et al., Lipids 1997, 32, 971).
Chia oil was identified a valuable source of branched-chain fatty acids with C15–C35 chain lengths. Thus, anteiso-22:0, anteiso-24:0, and anteiso-26:0 were unequivocally identified in that natural product. Chia seeds contain 0.4% branched-chain fatty acids, w/w of total fatty acids, or 32 mg branched-chain fatty acids in a food serving, surpassing other plant oils. It must be noticed that plant-derived branched-chain fatty acids are predominantly anteiso, in contrast with similar iso and anteiso levels in ruminant and fermented foods (Wang DH et al., J Agric Food Che. 2020, 68, 13871).
The unsaturated 11-methyloctadec-12-enoic and 12-methyloctadec-10-enoic acids were identified in the seed oil of Byrsocarpus coccineus (Connaraceae) (Spencer GF et al., Lipids 1979, 14, 72). A trans-monounsaturated branched-chain fatty acid, 8-methyl-trans-6-nonenoic acid, is characteristic of specific compounds, the capsaicinoids, found in fruits of the genus Capsicum (Solanaceae). These plants (bell pepper, chili pepper) are among the oldest cultivated plants, their pungent fruits being used as spices for over 6000 years. Capsaicinoids are synthesized by an enzymatic condensation of vanillylamine and a medium chain branched acid (Thiele R et al., J Agric Food Chem 2008, 56, 4219). More than 20 compounds, different only in the fatty acid structures, have been described.
The sponges contain also large quantities of C14 up to C30 fatty acids with branch as well as odd-chains (Carballeira N et al., Lipids 1989, 24, 229). As an example, a new structure (20-methyl-26:0) has been elucidated in the sponge Verongia aerophoba from the Canary islands (Nechev J et al., Eur J Lipid Sci Technol 2002, 104, 800) while some monomethyl polyunsaturated fatty acids were described in different marine sponges (24-methyl-5,9-pentacosadienoic acid or 2-methoxy-13-methyl-6-tetradecenoic acid) (Caballeira NM et al., J Nat Prod 2001, 64, 620) . Similarly, two new 2-methyl branched monoenoic very long chain fatty acids (2-methyl-24:1 n-7 and 2-methyl-26:1 n-9) were described in a marine sponge Halichondria panicea (Imbs AB et al., Chem Phys Lipids 2004, 129, 173). A new branched monounsaturated fatty acid (2-methyl-13-eicosanoic acid) has been described in a temperate clacisponge Leuconia johnstoni (Quevrain E et al., Lipids 2012, 47, 345).
It must be noticed that the similarity between the composition of the midchain branching pattern of fatty acids in some sponges and in bacteria suggests the presence of bacteria in these sponges. For Calcarea, the constant and prominent occurrence of iso– and anteiso-fatty acids (>40% of the total fatty acids in most species) suggests an origin of these compounds from the sponge cells rather than from bacterial lipids (Schreiber A et al., Chem Phys Lipids 2006, 143, 29). Another indication for a sponge cell origin of these compounds is the major presence (7-15 % of the total) of anteiso-nonadecanoic acid (16-methyloctadecanoic acid), an exotic compound that has not yet been reported as a major fatty acid in bacteria.
It has been found that Caenorhabditis elegans is able to synthesized iso-C15 and iso-C17 and that these branched-chain fatty acids are essential for the animal growth and development (Kniazeva M et al., PLoS Biol 2004, 2, E257). These results suggest that these fatty acids may play a potentially important role in other eukaryotes.
The occurrence of branched-chain fatty acids as major constituents in bacteria was first reported for Bacillus subtilis (Kaneda T, J Biol Chem 1963, 238, 1222). Branched-chain fatty acids of the iso and anteiso series occur widely in bacteria are of value in improving bacterial systematics. It should be noted that the fatty acid profile of bacteria with a branched-chain lipid type is affected by its growth conditions. The biosynthesis, function and taxonomic significance of branched-chain fatty acids in bacteria have been reviewed (Kaneda T, Microbiol Rev 1991, 55, 288).
12-Methyltetradecanoid acid, obtained from a deep-sea Streptomyces sp. through bioassay-guided isolation procedure, was found to have effective inhibition on larval settlement of the polychaete Hydroides elegans with EC50 value of 0.6 μg/mL (Xu Y et al., Mar. Biotechnol. 2009, 11, 495),
A new unsaturated methyl-branched fatty acid, 9-methyl-16:1(n-6) and the uncommon 11-methyl- 18:1(n-6) were found in the lipid extract of a new strain of bacterium Vibrio alginolyticus associated with the alga Cladophora coelothrix (Carballeira NM et al., Lipids, 1997, 32, 1271). Another novel methyl-branched fatty acid, 10-methyl-18:1(n-9), was found in the lipid extract of the marine fungus Microsphaeropis olivacea (Yu CM et al., Can. J. Chem., 1996, 74, 730).
The diffusible factor, which regulates the virulence in Xanthomonas campestris, likely as in several other bacterial species, was identified as cis-11-methyl-2-dodecenoic acid (Wang LH et al., Mol Bacteriol 2004, 51, 903).

cis-11-methyl-2-dodecenoic acid
Unexpectedly, this key component of microbial cell-cell communication systems is also able, as farnesoic acid, to regulate the morphological transition and virulence in a fungal pathogen, Candida albicans.
Branched chain fatty acids are also found in meat and milk from ruminants (e.g., cattle, goat, and sheep), which are derived from the cell membranes of bacteria leaving the rumen. Fermented soybean products such as natto, and ruminant milk and meat are the major source of these fatty acids in the human diet, with dairy and beef being the main source in occidental countries. These fatty acids in dairy products and beef range from 14 to 18 carbons, with branched 15:0 (iso 15:0, anteiso 15:0) and 17:0 (iso 17:0 and anteiso 17:0) making up to about 78% of the total.
iso 15:0 purified from a soy fermentation product was shown to exert potent anticarcinogenic effects in various cancer cell lines (Yang Z et al., Cancer Res 2000, 60, 505). More recently, iso- but not anteiso-branched chain fatty acids were shown to exert growth-inhibiting and apoptosis-inducing effects in MCF-7 human breast cancer cells (Vahmani P et al., J Agric Food Chem 2019, 67, 10042). High protein diets lead to increased levels of intestinal branched chain fatty acids, partly because the branched-chain amino acids leucine, isoleucine or valine are fermented by intestinal bacteria to fatty acids. They can be also elongated both in bacteria and in human tissues.
A review describes the occurrence of branched chain fatty acids, their dietary sources, their potential health effects, and the current state of knowledge concerning their mechanism(s) of action (Gozdzik P et al., Prog Lipid Res 2023, 90, 101226).
Branched methyl-substituted fatty acids of bacterial origin are commonly found in lake or marine sediments, decreasing rapidly with depth (Matsuda H et al., Geochim Cosmochim 1977, 41, 777). Long-chain monomethyl-branched anteiso acids were also identified in settlings particles and surface sediments from freshwater lakes where they may be useful molecular markers for lake acidity (Fukushima K et al., Org Geochem 2005, 36, 311).
10-Methyl octadecanoic acid (tuberculostearic acid) is present at the sn-2 position in the phosphatidylinositol mannosides found mainly in several Mycobacterium species (Gilleron M et al., J Biol Chem 2001, 278, 29880). Later, a similar position of tuberculostearic has been described in phosphatidylethanolamines from Mycobacterium tuberculosis (Horst B et al., J Lipid Res 2010, 51, 1017). The detection of this fatty acid in cerebrospinal fluid was proposed as a possibility for rapid and specific diagnosis of tuberculous meningitis. The presence of this fatty acid in sputum lipids was successively utilized for the diagnosis of tuberculous pneumonia (Larsson L et al., J Clin Microbiol 1987, 25, 893). 10-methyl nonadecanoic acid (phytomonic acid) is also found in Mycobacterium.
In animals, 17-methyl-6-octadecenoic and 17-methyl-7-octadecenoic acids were identified in the Australian mollusk Siphonaria denticulata (Carballeira NM et al. J Nat Prod 2001, 64, 1426).
An unusual complex and polyunsaturated fatty acid substituted with one hydroxyl and one aldehyde group has been described as a new polyene pigment, laetiporic acid, in the wood-rotting basidiomycete Laetiporus sulphureus (Weber RW et al., Tetrahedron lett 2004, 45, 1075). This orange pigment, with an UV-visible spectrum similar to that of carotenoids, bears an unprecedented decaene skeleton as part of its chromophore.
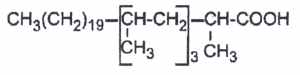
2 – Multimethyl branched acids are found mainly in bacteria.
Several dimethylated fatty acids (14 or 16 carbon atoms) with the first methyl substituent at carbon 2 or 4 have been isolated from a halophilic Bacillus species (Carballeira NM et al., J Nat Prod 2001, 64, 256).
Di- and tri-methylated fatty acids with 15 to 18 carbon atoms have been isolated from environmental subsurface sediments (Hedrick DB et al., Lipids 2008, 43, 843). These fatty acids may be of value for the knowledge of biomass and of the metabolic status of the viable microbial community.
A novel dimethylated fatty acid, 12,17-dimethyloctadecanoic acid, has been described in high concentration (16.3 % of total fatty acids) in an extremophile bacteria, Thermogemmatispora sp. (Vyssotski M et al., Lipids 2012, 47, 601). Dimethyl-branched fatty acids of this type are rare and the authors suggested that having this type of fatty acid as a major component could provide a useful taxonomic marker for Thermogemmatispora microorganisms.
Multimethyl branched acids are abundant in cell wall lipids of Mycobacteria, each methyl group being on even carbon atoms (2,4,6,8…from the methyl end). Thus, forming waxes and glycolipids (mycosides), several multibranched fatty acids are commonly found :
– mycolipanolic acid (2,4,6-trimethyltetracosanoic acid). It has been isolated from the lipids of human tubercle bacilli (Coles L et al., J Chem Soc 1968, 1541-4).
– mycoceranic acid(2,4,6-trimethyloctacosanoic)
– mycolipenic acid (2,4,6-trimethyl-trans-2-tetracosenoic acid)
– micolipodienoic acid (2,4,6-trimethyltetracosadienoic acid)
– mycocerosic acid (2,4,6,8-tetramethyl C32 fatty acid)
– phthioceranic acids which are hepta or octamethyl fatty acids, some of them being also hydroxylated (hydroxyphthioceranic acid).
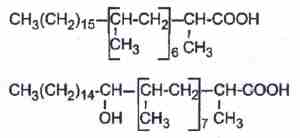
– dolichoic acids have been discovered is lipid extracts of human brain (Ward W et al., J Lipid Res 2007, 48, 1457). These acids, derived from dolichols, contain 14-20 isoprene units. Future work remains in determining the biosynthetic pathway for these acids and elucidating their function within the brain.
The analysis of mycolic, mycocerosic and mycolipenic acids and phthiocerol compounds in old skeletons from a human settlement at Atlit-Yam, Israel (~9,000 year-old) and from an extinct Bison antiquus from Natural Trap Cave, Wyoming (~17,000 year-old) have provided evidence for tuberculosis in these landmark specimens (Lee OY et al., Tuberculosis 2015 S1472-9792). The hypothesis that this disease evolved as a zoonosis, before transfer to humans has been previously discussed (Lee OY et al., PLoS One. 2012;7(7):e41923).
Branched acids but with shorter chains are also found in the depot fats of ruminant animals, in sebum and animal waxes (wool-wax). Ruminants produced a huge variety of C10-C18 acids with one to four branched methyl groups, they were detected in lamb adipose tissue. Multimethyl branched acids are also dominant in the uropygial waxes of the birds preen gland. Curiously, they were also identified in the lipid depot in the head of marine animals, location involved in their echo-locating abilities.
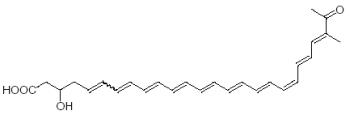
Unusual branched fatty acids have been isolated as minor components from the glycolipids (GL) fraction of freshwater sponges (Dembitsky VM et al., Chem Phys Lipids 2003, 123, 117).It is possible that these neo acids could be of cyanobacterial origin. An example of one of them with the longest carbon chain is shown below.
Others have isoprenoid structures, thus coming from the diterpene phytol derived from chlorophyll and not by the de novo pathway. Among them, two are found in marine organisms, in geological sediments but, one of them (phytanic acid or 3,7,11,15-tetramethyl hexadecanoic acid) is present in human diet or in animal tissues where it may be derived from chlorophyll in plant extracts. Phytanic acid is present in cow milk fat but its concentration is affected by feed composition (Che BN et al., J Agric Food Comp 2013, 61, 225).
Two very unusual phytyl esters were obtained from the extract of the hornwort Megaceros flagellaris (Bryophyte, Anthocerothae). The fatty acid moiety comprises 3,7,11,15-tetramethyl-16:1 or 3,7,11,15-tetramethyl-16:0, which is esterified to the corresponding tetramethyl unsaturated (16:1) alcohol (Buchanan MS et al., Phytochemistry, 1996, 41, 1373).
Phytanic acid derives from the corresponding alcohol, phytol, and is oxidized into pristanic acid.
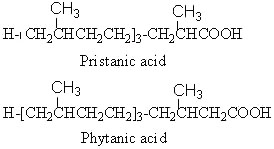
Pristanic acid was first isolated from butter (Hansen RP et al., Biochemical Journal 1964, 93, 225). The name of the substance is derived from pristane (2,6,10,14-tetramethylpentadecane), the corresponding hydrocarbon which was isolated from shark (pristis in Latin). It is also found in the lipids from many sources such as sponges, crustacea, milk fats, animal depot fat but also in petroleum samples.
Phytanic acid characterizes a precise human pathology, the Refsum’s syndrome. This inherited neurological disorder (Refsum S, Acta Psychiat Scand Suppl 1946, 38, 9) is characterized by a accumulation of phytanic acid, normal metabolite of phytol, in blood and tissues (Klenk E et al., Hoppe Seyler’s Z Physiol Chem 1963, 333, 133). The disorder was later related to deficiency in the alpha-oxidation pathway in the liver (Herndon JH et al., J Clin Invest 1969, 48, 1017; review in : Mukherji M et al., Prog Lipid Res 2003, 42, 359-376). Both phytanic acid and pristanic acid have been shown to activate the peroxisome proliferator-activated receptor a (PPARa) in a concentration-dependent manner (Zomer AW et al., J Lipid Res 2000, 41, 1801). The presence of phytanic acid in food is related to the fatty alcohol phytol, contained in chlorophyll, ingested by ruminants and fish. The bioconversion of phytol into phytanic acid is effective in the rumen and in the marine environment. Thus, milk, other dairy products and meat from ruminants as well as fish contain the highest concentrations of phytanic acid in the range of 100-500 mg/100 g lipids. Persons suffering from the Refsum syndrome must restrict to the minimum the absorption of dairy products and ruminant flesh.
Freshwater sponges contain polymethyl branched fatty acids such as 4,8,12-trimethyltridecanoic, phytanic and pristanic acids. These acids may have chemotaxonomical significance for both marine and freshwater sponges (review in : Dembitsky VM et al., Chem Phys Lipids 2003, 123, 117). The isoprenoic 4,8,12-trimethyltridecanoic was found to be always present in the marine calcareous sponges (Calcarea) but in minor amounts (Schreiber A et al., Chem Phys Lipids 2006, 143, 29), this acid being presumed to be derived from phytol, a degradation product of chlorophyll. Phytanic acid present in immature (recent) sediments is thought to derive from phytol, a supposition backed by stereochemical studies (Maclean I et al., Nature 1968, 218, 1019).
Few sources, including sponges, contain branched polyunsaturated fatty acids (Rezanka T, Prog Lipid Res 1989, 28, 147). As an example, freshwater Demospongia (Spongillidae) were shown to contain di-, tri-, and tetramethyl substituted dienoic, tetraenoic, and hexaenoic fatty acids (Rezanka T et al., J Nat Prod 2002, 65, 709). A review of these rare polyenoic fatty acids was released by Dembitsky VM et al (Chem Phys Lipids 2003, 123, 117).
Geranylgeranoic acid (GGA; all-trans 3,7,11,15-tetramethyl-2,6,10,14-hexadecatetraenoic acid) has been detected in several plants (Shidoji Y et al., J Lipid Res 2004, 45, 1092). Highest amounts were detected in schisandra fruit (Schisandra chinensis), turmeric (Curcuma longa) and licorice (Glycyrrhiza uralensis) rhizomes. Interestingly, the same authors have shown that GGA was able to induce apoptosis in a human hepatoma-derived cell line, Later, it was shown that hepatic monoamine oxidase B is involved in the biosynthesis of GGA which can induce cell death in human hepatoma-derived cell lines by noncanonical pyroptosis, one of the mechanisms of sterile inflammatory cell death (Shidoji Y, J Lipid Res 2023, 64, 100396). A derivative of GGA, peretinoin (4,5-didehydroGGA), was observed to inhibit cell proliferation, upregulate albumin gene expression, downregulate α-fetoprotein gene expression, and induce differentiation into hepatocytes when added to a culture system of human hepatoma-derived cell lines (Yamada Y et al., Mol Carcinog 1994, 10, 151).
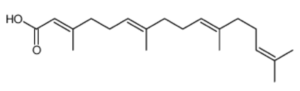 Geranylgeranoic acid
Geranylgeranoic acid
Some isoprene derivatives (sesquiterpenes) synthesized by invertebrates from farnesoic acid have important endocrinological functions (juvenile hormones) such as molting, reproduction and metamorphosis.
A lactone derivative of a terpenoid fatty acid hydroxylated on the carbon 3 (Vittatalactone) has been determined as a pheromone emitted by the male striped cucumber beetles, Acalymma vittatum, an insect which causes a major damage to cucurbit crops in North America (Morris BD et al., J Nat Prod 2005, 68, 26).
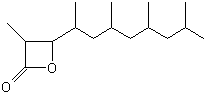
Vittatalactone
Addition of isoprene acids to proteins (prenylation), discovered in 1984 (Schmidt RA et al., J Biol Chem 1984, 259, 10175), is an important post-translational modification of proteins which has been recognized as a key physiological process for facilitating cellular protein-protein interactions and membrane-associated protein trafficking. Protein prenylation occurs by the covalent addition of two types of isoprenoids, farnesyl pyrophosphate (a 15-carbon sesquiterpene) or geranylgeranyl pyrophosphate (a 20-carbon diterpene), to cysteine residues at or near the terminal carboxyl group . The largest family of prenylated proteins are the intracellular GTP-binding proteins that transduce extracellular signals into intracellular changes via downstream effectors (McTaggart SJ, Cell Mol Life Sci 2006, 63, 255).
Some isoprenoid fatty acids with conjugated double bonds are known. In this group, the most interesting is retinoic acid which derives from retinol but is synthetized ultimately from b-carotene (provitamin A). It has important functions in cell regulation.
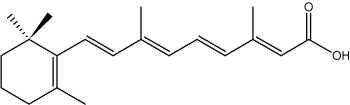
all-trans-Retinoic acid
Abscissic acid, a methylated derivative of retinoic acid, plays a variety of roles in plant physiology. It is ubiquitous in plants, including algae.
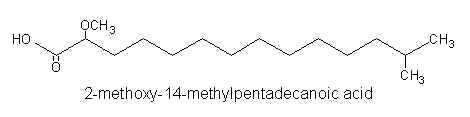
Iso or anteiso alpha-methoxylated C15-C16 fatty acids were reported in phospholipids of a Caribean sponge Amphimedon complanata (Carballeira NM et al. Lipids 2001, 36, 83).
Thus, 2-methoxy-13-methyltetradecanoic acid, 2-methoxy-14-methylpentadecanoic acid, and 2-methoxy-13-methyl pentadecanoic acid were identified by gas-liquid chromatography and mass spectrometry.
It has been suggested that these compounds have originated from a novel bacteria in symbiosis with the sponge.
Devenez membre et participez au développement de la Lipidomique au XXIème siècle.
S'inscrire