
Sterols may be found either as:
– free sterols,
– acylated (sterol esters),
– alkylated (steryl alkyl ethers),
– sulfated (sterol sulfate),
– linked to a glycoside moiety (steryl glycosides)
– which can be itself acylated (acylated sterol glycosides)
– or sulfated (sulfated steryl glycosides)
– or chlorinated (chlorinated cholesterol)
Sterols are forms of steroids with a hydroxy group at position three and a skeleton derived from cholestane.
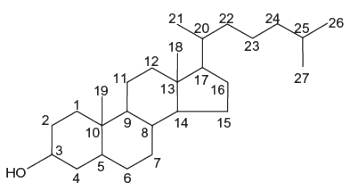
More than 250 sterols have been described but all have in common the tetracyclic sterane backbone consisting of 17 carbon atoms and an obligate OH moiety on C-3. Their structural diversity results from variations in the total number of carbon atoms (i.e. 27 to 32 carbon atoms including an alkyl residue of 8 to 10 carbon atoms on C-17 (the side chain), as well as zero to four double bonds. Their structures differ also in the number and position of double bond(s), the number of methyl substituents on C-4, the absence or presence of a methyl group on C-14 and several other structural specificities (Schlag S et al., Food Chem 2024, 140778).
The true sterols are accompanied by closely related compounds (both structurally and biosynthetically) such as other tetracyclic (e.g. butyrospermol, tirucallol) and pentacyclic (e.g. amyrins) triterpene-derived compounds with an OH group on C-3 (triterpenoids).
Butyrospermol is a protolimonoid extracted from shea (Euphorbia). It is also present in Camellia oil, which is obtained from camellia seeds (Camellia oleifera and Camellia japonica). It has antifeedant, antitumor. and anti-inflammatory activities (Li Y et al., Food Chem 2025, 472, 142931).
 Butyrospermol
Butyrospermol
Tirucallol is a natural product found in Camellia sinensis and Glycine max.
The amyrins are three closely related natural chemical compounds of the triterpene class isolated from a variety of plant sources such as epicuticular wax.
Sterol biosynthesis is nearly ubiquitous among eukaryotes, it is almost completely absent in prokaryotes. They are not found in Archaea and the proven occurrences in bacteria are sparsely distributed and yield a limited array of products. The proteobacterium Methylococcus capsulatus and the planctomycete Gemmata obscuriglobus (Pearson A et al., PNAS 2003, 100, 15357) and some members of the myxobacteria are proven steroid-producing bacteria. As a result, the presence of diverse steranes (saturated 4-cycle skeleton) in ancient rocks is used as evidence for eukaryotic evolution. Thus, the concentration of eukaryotic steranes, relative to bacterial hopanes, may provide basic information about the ecological relevance of Precambrian algae. It has been shown that the relative abundance as well as diversity of steranes dramatically increased in the brief interlude between around 659–645 Ma (neoprotozoic period), heralding the rise of planktonic algae as important primary producers in the oceans at that time (Gueneli N et al., PNAS 2018, 115, E6978).
More recently, abundant protosteroids were discovered in sedimentary rocks from northern Australia of mid-Proterozoic age.
 Protosterol
Protosterol
The protosteroids reveal an ecologically prominent ‘protosterol biota’ that was widespread and abundant in aquatic environments from at least 1,640 to around 800 million years ago and that probably comprised ancient protosterol-producing bacteria and deep-branching stem-group eukaryotes (Brocks JJ et al., Nature 07 June 2023).
Structure of sterols with carbon numbers
Sterols are derived from the same squalene precursor as hopanoids but, in marked contrast, they are known to have an oxygen-dependent biosynthesis beginning with the formation of the first intermediate, 2,3- oxidosqualene. There is a close connection between modern-day biosynthesis of particular triterpenoid biomarkers and presence of molecular oxygen in the environment. Thus, the detection of steroid and triterpenoid hydrocarbons far back in Earth history has been used to infer the antiquity of oxygenic photosynthesis (Summons RE et al., Phil Trans R Soc B 2006, 361, 951). It has been hypothesized that increased levels of O2 in the atmosphere not only made the evolution of sterols possible, but that these sterols may in turn have facilitated the birth of complex organisms (the eukaryotes) (Chen LL et al., Biochem Biophys Res Comm 2007, 363, 885), likely in providing them with an early defense mechanism against O2 (Galea A et al., Free Rad Biol Med 2009, 47, 880; Brown AJ et al., Evolution 2010, April 14).
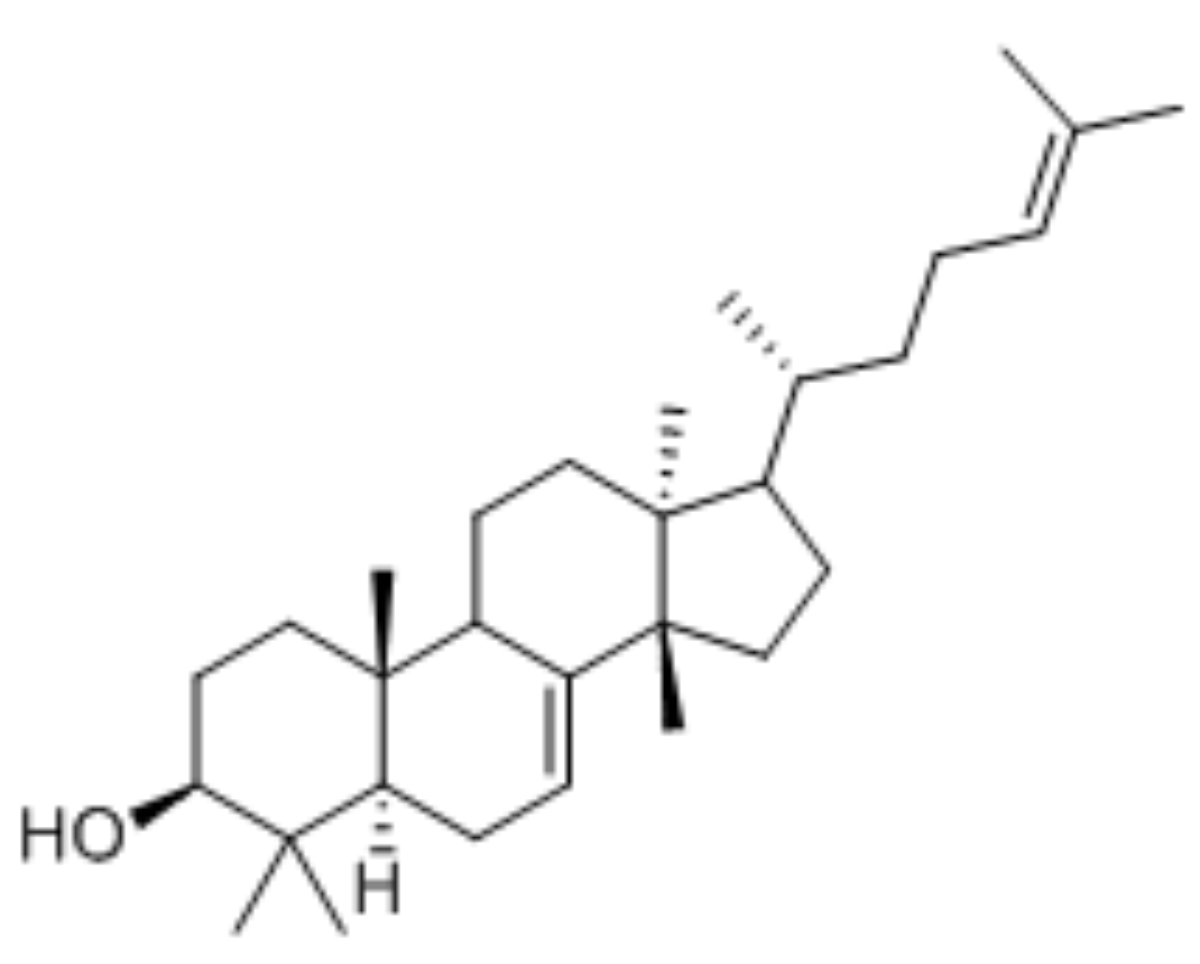
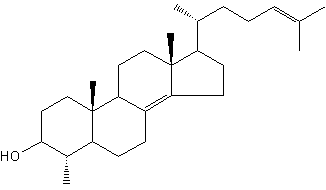
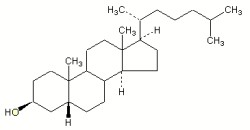
Sterols form an important group among the steroids. Unsaturated steroids with most of the skeleton of cholestane containing a 3β-hydroxyl group and an aliphatic side chain of 8 or more carbon atoms attached to position 17 form the group of sterols.
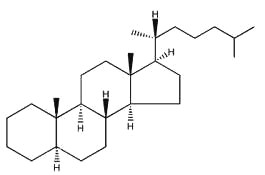
5α-cholestane
They are lipids resistant to saponification and are found in an appreciable quantity in all animal and vegetal tissues. Furthermore, cholestane may be considered as a biological marker compound valuable in the assessment of marine sediment maturity, even after hundreds of millions of years (Mackenzie AS et al., Science 1982, 217, 491). Sterols may include one or more of a variety of molecules belonging to 3-hydroxysteroids, they are C27-C30 crystalline alcohols (in Greek, stereos, solid). These lipids can be classed also as triterpenes, as they derive from squalene which gives directly by cyclization, unsaturation and 3b-hydroxylation, lanosterol in animals or cycloartenol in plants.
Cholesterol (Greek, chole, bile), in the tissues of vertebrates, is the main sterol (C27 alcohol), particularly abundant in adrenals (10%, w/w), nervous tissues (2%,w/w), liver (0.2%,w/w) and gall stones. The vertebrate brain is the most cholesterol-rich organ, containing roughly 25% of the total free Cholesterol present in the whole body. Its fundamental carbon structure is a cyclopentanoperhydrophenanthrene ring (also called sterane).
A limited number of studies have shown the presence of trace amounts of cholesterol in plants. The presence of cholesterol was assessed in onion (Allium cepa), spring onion (Allium fistulosum) using precise GC-MS techniques at concentrations of 23.4 and 7.3 mg/100 g dry weight, respectively (Prayitno V et al., J Food comp Anal 2025, 147, 108061).
HISTORY OF CHOLESTEROL
As early as the 18th century, doctors and chemists BOERHAAVE H. (1743), HALLER A. (1769), CADET L. (1751) thought that gallbladder stones were composed of the same inflammable oily material as bile.
The Italian VALLISNERI A. (1661-1730), Professor of Medicine in Padua, observed that gallstones dissolved in a mixture of wine alcohol and turpentine.In Volume II of the « Histoire et Mémoire de la Société Royale de Médecine » (1777-1778), it is written that “spirits of wine dissolve biliary concretions very well. Mr. POULLETIER DE LA SALLE, our colleague, very well versed in all branches of physics, and mainly in chemistry, obtained, by filtering spirits of wine, loaded with the matter of these concretions, a large quantity of salt which closely resembles sedative salt.”
Let us also point out that this discovery was again cited by FOURCROY A.F. (1793) and by THOURET M.A. (1790) in the « Histoire et Mémoires de la Société Royale de Médecine ».
The English scientist POWELL R. (1757-1834) reports that the material of biliary concretions was different from spermaceti, not only in its temperature of fusion, but also in the fact that it was dissolved by sulfuric ether and, above all, in the fact that only spermaceti underwent transformation after the action of a potash solution. Thus, POWELL correctly demonstrated, fifteen years before CHEVREUL M.E., that cholesterol is an unsaponifiable substance.
CHEVREUL M.E. provided the decisive impetus by identifying “cholesterine” and truly revolutionized the chemistry of fatty substances.
The seminal article is the fifth memoir devoted to his “Recherches chimiques sur plusieurs corps gras, et particulièrement sur leurs combinaisons avec les alcalis”. This memoir established that the substance of gallstones is a simple, unsaponifiable substance, fusible at 137°. “I will call cholesterine, de χολη bile et στερεος solide, the crystallized substance of human gallstones.”
There are before and after CHEVREUL in the history of fatty acid chemistry, just as there are before and after CHEVREUL in the history of sterol chemistry. The term “cholesterin” was not abandoned in France until the end of the 1920s and 1930s.
It is also legitimate to think that CHEVREUL’s work on cholesterol would not have been what it was if POULLETIER’s and especially FOURCROY’s work had not preceded it.
Later, it remained to highlight cholesterol in the healthy human organism. This was done for the first time in 1824 in bile, by CHEVREUL.
In 1829, pharmacist LECANU L.R. published a “note on the existence of cholesterol in egg yolk oil.” In 1828, DENIS P.S. presented his “experimental research on human blood, considered in a healthy state,” a liquid in which he first reported the presence of cholesterol.
It was not until 1833 that Félix BOUDET F. discovered and demonstrated the normal presence of cholesterol in the blood.
In 1834, Couerbe J.P. (1807-1867) presented the results of his research on the composition of the brain to the French Academy of Sciences. He clearly demonstrated that cholesterol is a major component of the nervous system and that it appears to be produced by it.
Thanks to the works of chemists in the 18th and early 19th centuries, cholesterol was recognized as widespread in animal science. It remained for medical biologists, physiologists, and pathologists to recognize its role in the healthy body and in the development of certain pathological lesions.
In 1885, Liebermann C. (1842-1914) developed a color reaction that would allow for the sensitive measurement of cholesterol. In 1889, H. Burchard H. slightly modified this reaction by recommending the dissolution of cholesterol in chloroform. He added acetic anhydride and concentrated sulfuric acid. A beautiful green hue appeared after thirty minutes.
This color reaction was named the Liebermann-Burchard reaction and was used for decades for routine cholesterol measurement.
In 1896, HURTHLE K isolated two cholesterol esters from serum: cholesterol palmitate and stearate.
GRIGAUT A., in the laboratory of CHAUFFARD A. (1855-1932), wrote in his 1913 thesis: “In healthy humans, under normal dietary conditions, cholesterol levels are generally between 1.40 and 1.90 grams per liter, and 1.60 grams can be considered the average cholesterol level.”
These figures are similar to those accepted today.
Within a few years, the techniques of cholesterol measurement had become sufficiently reliable and reproducible to enter routine medical practice.
A detailed history of the discovery of cholesterol and its implications for medicine can be found in the Feltgen K.’s medical doctoral thesis (https://drive.google.com/file/d/1ZwTryw2flnXPmgCftAfqmKsAqXwQB4jt/view).
The correct formula (C27H46O) was proposed in 1888 by F. Reinitzer but structural studies from 1900 to 1932, mainly by H.O. Wieland “on the constitution of the bile acids and related substances” (Nobel Prize Chemistry 1927) and by A.O.R. Windaus on “the constitution of sterols and their connection with the vitamins” (Nobel Prize Chemistry 1928) led to the exact steric representation of cholesterol. In 1936,
Callow RK and Young FG have designated steroids all compounds chemically related to cholesterol. The main steps of cholesterol research have been reviewed up to the year 2000 (Vance DE et al., Biochim Biophys Acta 2000, 1529, 1).
It must be noticed that the central role of cholesterol in atherogenesis has been proposed by a young Russian pathologist in Saint Petersburg in 1913 (Steinberg D, J Lipid Res 2013, 54, 2946).
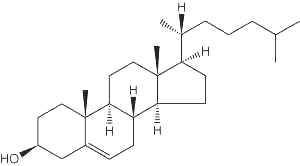
Cholesterol
Cholesterol is found in high concentrations in animal cell membranes, typical concentrations (expressed as molar percentage of total lipids) being about 30 mol%, ranging up to 50 mol% in red blood cells and as high as 80 mol% in the ocular lens membranes (Li LK et al., J Lipid Res 1985, 26, 600). Consequently, cholesterol has numerous functions in membranes ranging from metabolism, as a precursor to hormones and vitamins, to providing mechanical strength and a control of the phase behavior of membranes (Rog T et al., Biochim Biophys Acta 2009, 1788, 97). It became clear that the key role of cholesterol in the lateral organization of membranes and its free volume distribution seems to be involved in controlling membrane protein activity and “raft” formation (review in Barenholz Y, Prog Lipid Res 2002, 41, 1). At the cellular level, cholesterol may be replaced to some extent by some other sterols with minor modifications of the side chain (campesterol, β-sitosterol) (Xu F et al., PNAS 2005, 102, 14551). More specifically, it is admitted that the maintenance of brain cholesterol homeostasis is essential for brain functioning and development, and its dysregulation is associated with Alzheimer’s disease, neuroinflammation and oxidative damage (Staurenghi E et al., Antioxidants 2024, 13, 435).
While structurally similar, cholesterol is far more absorbed from food in intestine (>50%) than phytosterols (<2%). It may be hypothesized that phytosterols are poor substrates of intestinal acyl-CoA: cholesterol acyltransferase 2, and thus minimal phytosterol esters are formed and packed into chylomicrons, leading to their low absorption (Chen Z et al., Food Chem July 2024, 140300).
Cholesterol is abundant in the femoral gland of the male lizard Acanthodactylus boskianus which uses it as a scent marking pheromone to establish dominance hierarchies (Khannoon ER et al., Chemoecology 2011, 21, 143).
In addition to these roles, cholesterol can form ester linkages with a class of secreted polypeptide signaling molecules encoded by the hedgehog gene family. These proteins function in several patterning events during metazoan development (Mann R et al., Biochim Biophys Acta 2000, 1529, 188).
People with mutations in the enzyme which converts 7-dehydrocholesterol into cholesterol (a reductase), show elevated levels of 7-dehydrocholesterol. These people suffer from the Smith–Lemli–Opitz syndrome which can induce physical, cognitive and behavioural symptoms of varying severity (Wikipedia). Prenatally, the syndrome may be diagnosed upon finding an elevated 7-dehydrocholesterol/total sterol ratio in fetal tissues, or increased levels of 7-dehydrocholesterol in amniotic fluid.
7-Ketocholesterol, one of the cholesterol oxidization products, It is both provided by food and produced endogenously. 7-Ketocholesterol appears to significantly contribute to the development of age-related diseases (cardiovascular diseases, age-related macular degeneration, and Alzheimer’s disease), chronic inflammatory bowel diseases and to certain cancers.
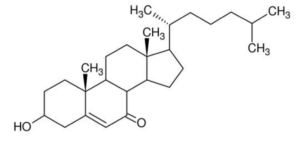
7-Ketocholesterol
Studies have also shown that it has anti-viral activities, including on SARS-CoV-2, Furthermore, it increased in the serum of moderately and severely affected COVID-19 patients. Thus, 7-ketocholesterol could possibly constitute a lipid biomarker of COVID-19 outcome and counteracting its toxic effects with adjuvant therapies might have beneficial effects in COVID-19 patients (Ghzaiel I et al., J Steroid Biochem Mol Biol. 2021, 212, 105939). Further studies have explored the prognostic value of plasma 7-ketocholesterol in sepsis (Zhang Y et al., Clin Chim Acta 2023, 548, 117467).
Cholesterol extracted from organically preserved Ediacaran macrofossils unambiguously was used to clarify their phylogeny (Bobrovskiy I et al., Science 2018, 361, 1246). Indeed, the enigmatic Ediacara biota (571 million to 541 million years ago) represents the first macroscopic complex organisms in the geological record and may hold the key to our understanding of the origin of animals. Ediacaran macrofossils are as “strange as life on another planet” and were interpreted as marine animals or giant single-celled protists or terrestrial lichens. Thanks to these new results make these iconic members of the Ediacara biota the oldest confirmed macroscopic animals in the rock record.
Olesoxime (TRO19622) is a synthetic cholesterol-like structure and belongs to the cholesterol-oxime family of mitochondrial pore modulators. That compound is an experimental drug as a treatment for a range of neuromuscular disorders (Bordet T et al., J Pharmacol Exp Therapeutics 2007, 322, 709).
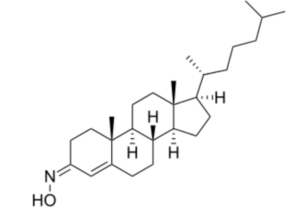
Olesoxime
Sponges, a primitive group of multicellular organisms (Poriphera), represent the richest source of bizarre sterols found in nature. Most sponges have the general sterol structure found in animals, plants, and fungi., i.e. cholesterol and sterols, but bearing one to three extra carbon atoms at C24. These side chains have been isolated with such unusual features as quaternary alkyl groups, cyclopropane and cyclopropene rings, allenes, and even acetylenes (Giner JL, Chem Rev 1993, 93, 1735).
24-Isopropylcholesterol is abundant and characteristic (with its analogue unsaturated at C22-C23) of the class Demospongiae. This sterol is absent in “true animals”, the eumetazoans (cnidarians and bilaterian animals). This demosponge sterane is abundant in sediment dating from the Neoproterozoic era (1,000-542 million years) and is the oldest evidence for animals in the fossil record (Love GD et al., Nature 2009, 457, 718).
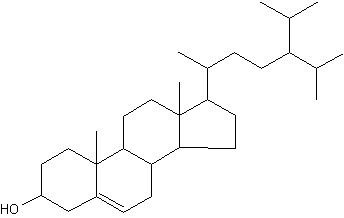
24-Isopropylcholesterol
Clionasterol, a 29 carbon structure compound was isolated from the green alga Halimeda macroloba collected in Kenya (Dzeha T et al., Western Indian Ocean J 2003, 2, 157). It was previously isolated from the Indian marine red alga Gracilaria edulis (Das B et al., Phytochem 1992, 31, 1054), it was also detected in Spirulina.
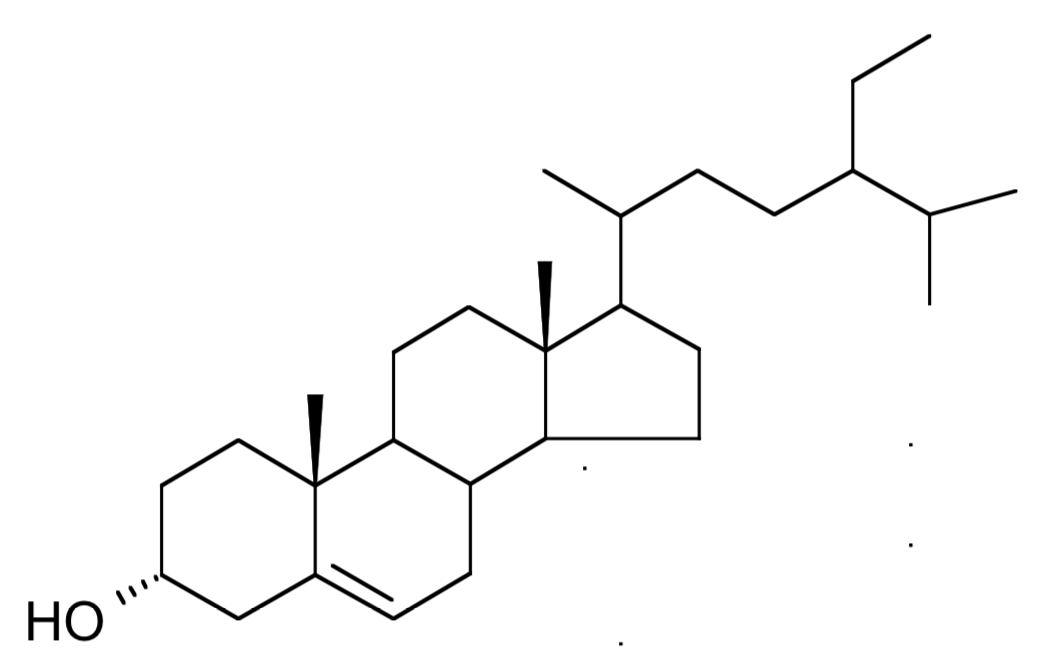 Clionasterol
Clionasterol
A new acetylenic derivative of cholesterol, gelliusterol E, was isolated from the Red sea sponge Callyspongia aff. implexa and showed that it is a potent inhibitor of the primary infection by Chlamydia trachomatis, an obligate intracellular Gram-negative bacterium (Abdelmohsen UR et al., Planta Med 2015, 81, 382).
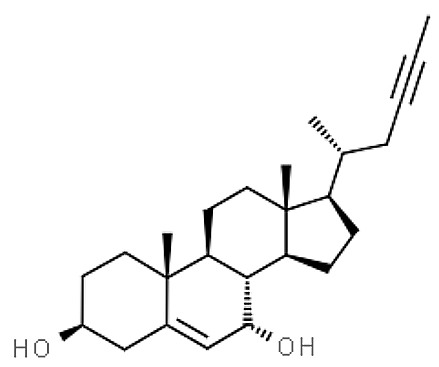
Gelliusterol
Sponges of the genus Theonella have fascinated the scientific community as they have proved to be a prolific source of original sterols. The main class of sterols isolated from these sponges, particularly from Theonella swinhoei (collected at the Solomon Islands) and Theonella conica, are 4-exo-methylene sterols, relatively rare metabolites in nature. The biosynthetically unusual 4-exo-methylene group arises from the oxidative demethylation of the 4,4-dimethyl precursor followed by oxidation and dehydration of the primary alcohol affording the 4-exo-double bond (Kho E et al., J Org Chem 1981, 46, 1836). A majority of these unique chemical compounds are characterized by potent and interesting biological activities such as antimicrobial, cytotoxic, and in some cases by modulating activity towards metabolic nuclear receptors (Festa C et al., Mar Drugs 2023, 21, 291). Among 4-exo-methylene sterols, theonellasterol is a typical compound largely studied.
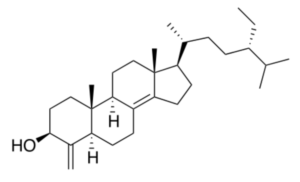 Theonellasterol
Theonellasterol
Among the large list of sterols with cyclopropane ring, Nicasterol was identified in a Demospongiae, Calyx nicaensis.
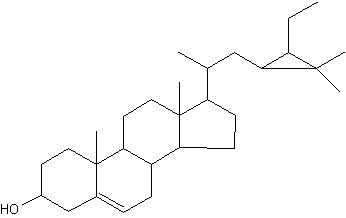
Nicasterol
While cholesterol was considered to be nearly absent in vegetal organisms, its presence is now largely accepted in higher plants. It can be detected in vegetal oils in a small proportion (up to 5% of the total sterols) but remains frequently present in trace amounts. An unusual relatively high content of cholesterol was described in camelina oil (about 200 mg per kg) (Shukla VKS et al., JAOCS 2002, 79, 965). However, several studies have revealed the existence of cholesterol as a major component sterol in chloroplasts, shoots and pollens. Furthermore, cholesterol has been detected as one of the major sterols in the surface lipids of higher plant leaves (rape) where he may amount to about 72% of the total sterols in that fraction (Noda M et al., Lipids 1988, 23, 439). Cholesterol is also dominant in most all Rhodophyceae algae, it is the only sterol presesnt in Laurencia paniculata (Al Easa H et al., Phytochemistry 1995, 39, 373).
|
|
|
|
In late-step synthesis of cholesterol, discrete oxidoreductive and/or demethylation reactions occur, which start with the common precursor lanosterol. Lanosterol is also found as a major constituent of the unsaponifiable portion of wool fat (lanoline) : about 15%. Our study identifies lanosterol as a key molecule in the prevention of lens protein aggregation and points to a novel strategy for cataract prevention and treatment ( Zhao L et al., Nature 2015, 523(7562), 607). Thus, it was shown that lanosterol treatment could reduce cataract severity and increase transparency in dissected rabbit cataractous lenses in vitro and cataract severity in vivo in dogs. More recently, it was demonstrated that activated lanosterol synthase pathway in lens is protective for lens transparency in cortical cataract (Shen X et al., J Ophthalmol 2018, 2018, 4125893).
It has been shown that the bacterium (planctomycete), Gemmata obscuriglobus, is able to synthesized lanosterol and its uncommon isomer, parkeol (Pearson A et al., PNAS 2003, 100, 15357). No subsequent modifications of these sterols were observed.
Several lanosterol derivatives have been identified in methanotrophic bacteria. The most abondant derivative, 4-methylcholestan-8(14),24-dien-3β-ol, has been found first in Methylococcus capsulatus (Bouvier P et al., Biochem J 1976, 159, 267) and later in other similar bacteria.
4-Methylcholestan-8(14),24-dien-3β-ol
Several compounds with the lanostane nucleus (ganoderates, lucidenates) have been isolated from a mushroom Polyporaceae (Ganoderma lucidum) (Boh B et al., Biotechnol Ann Rev 2007, 13, 265). These sterols could be related to the use of that mushroom in the traditional Chinese and Japanese medicine to cure several pathologies (hypertension, hypercholesterolemia, hepatitis, gastritis, diabetes, bronchitis, and cardiovascular problems) (Lee I et al., J Nat Prod 2010, 73, 172). One of several compounds named ganoderic acid A is shown below.
One of the ganoderic acids from Ganoderma lucidum
Animal tissues contain in addition to cholesterol small amounts of 7-dehydrocholesterol which, on UV irradiation, is converted to vitamin D3 (cholecalciferol). The amount of 7-dehydrocholesterol in milk has been related to the exposure of cows to sunlight and thus may an indication of living conditions for farm animals (Nádvorníková J et al., J Food Comp Anal 2023, 122, 105471).
Desmosterol (24-dehydrocholesterol), an intermediate between lanosterol and cholesterol, has been implicated with myelination processes. While high desmosterol levels could be detected in the brain of young animals (Paoletti R et al., J Am Oil Chem Soc 1965, 42, 400) no desmosterol was found in the brain of adult animals. It is also known as an abundant membrane component in some mammalian cells, such as spermatozoa and astrocytes (Lin DS et al., J Lipid Res 1993, 34, 491 – Mutka AL et al., J Biol Chem 2004, 279, 48654). Inability to convert desmosterol to cholesterol leads to the human disorder desmosterolosis (a severe developmental defect and cognitive impairment) (Waterham HR et al., Am J Hum Genet 2001, 69, 685). Desmosterol and 22-dehydrocholesterol are present in high concentrations in red algae.
In microalgae, sterols usually possess C27–C29 skeletons, with differences in alkylation at C-24 and double bonds in the nucleus (D5, D8, D8(14)) and side chain (D22, D24, D24(28). Dinoflagellates are unusual in terms of steroid composition, as they often contain sterols with additional methyl groups C-4 and C-23.
Gorgosterol was discovered by Bergmann in 1943 (Bergmann W et al., J Org Chem 1943, 8, 271) and named after the coral like animals from which it was isolated. It was later found that gorgosterol is actually produced by zooxanthellae (intracellular photosynthetic dinoflagellate symbionts). The original structure of gorgosterol, which bears an unusual C-23 methyl group and a cyclopropane ring in the side chain contributed greatly to the renewed interest in marine sterols.
Gorgosterol
Several similar compounds named gorgostane steroids have been isolated from marine organisms and consist of 30 carbon atoms with a characteristic cyclopropane moiety. The structural diversity, selectivity for marine organisms, and biological effects of gorgostane steroids have generated considerable interest in the field of drug discovery research (Abdelkarem FM et al., Marine Drugs 2022, 20, 139). For example, dinosterol (4α,23,24-trimethyl-5α-cholest- 22E-en-3β-ol) is typically found in dinoflagellates where it is generally accepted as a reliable biomarker (Boon JJ et al., Nature 1979, 277, 125).
Dinosterol
23-Methyl sterols have been also reported in diatoms and their sterane equivalents were also unambiguously identified in sediments and petroleum from the late Jurassic onwards (Rampen S et al., Org Geochem 2009, 40, 219).
Oxysterols : An important oxysterol, 24S-Hydroxycholesterol, is an enzymatically oxidized product of cholesterol mainly synthesized in the brain. It was detected in 1953 in horse brain and named “cerebrosterol” (Ercoli A et al., J Am Chem Soc 1953, 75, 3284). It was proposed that this oxysterol could be a biochemical marker for Alzheimer’s disease (Lütjohann D et al., J Lipid Res 2000, 41, 195). Furthermore, 24S-Hydroxycholesterol is reported to be protective against b-amyloid peptide, the amyloidogenic peptide found in plaques in Alzheimer disease brain (Brown J et al., J Biol Chem 2004, 279, 34674).
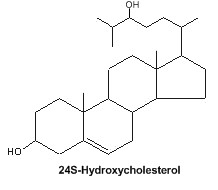
It has been shown that fluctuations in the levels of cholesterol oxidation products may correlate with the onset of Alzheimer’s disease and Parkinson disease (review in : Marwarha G et al., Exp Gerontol 2015, 68, 13).
Delta(7)-dafachronic acid, or (5α)-3-oxocholest-7-ene, a member of the class of dafachronic acids, is a cholestane derivative in which the methyl group at position 26 has been oxidised to the corresponding carboxylic acid. It could play an important role in the diet-mediated longevity enhancement.
A review of the other oxysterols is given elsewhere. Various aspects of oxysterols biology, mainly in bile acid metabollism, has been reviewed (Crosignani A et al., Clin Chim Acta 2011, 412, 2037).
Starfishes contain a great number of polar steroids (oxysterols) characterized by numerous hydroxylations which have no counterpart in the animal kingdom. As an example, the structure of a 5α-cholestane-hexaol present in a Far Eastern starfish, Henricia leviuscula, is given below (Ivanchina NV et al., J Nat Prod 2006, 69, 224).
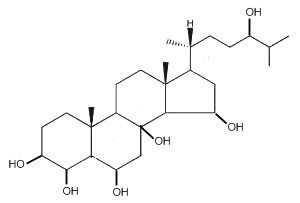
5α-Cholestane-hexaol
We had the opportunity to study Alcyonium sp. from . Six known alcyopterosins and three new ones (1–3) were obtained, A highly oxidized steroid, alcyosterone, has been isolated from the Antarctic soft corals Alcyonium living in deep-water communities near Shag Rocks in the Scotia Arc of Antarctica (Anne-Claire D. Limon et al., Mar Drugs 2022, 20(9), 576). The metabolites were screened in a number of anti-infective assays and several showed promise against Clostridium difficile and Leishmania donovani, the causative agent of leishmaniasis, a disfiguring disease that can be fatal if not treated.
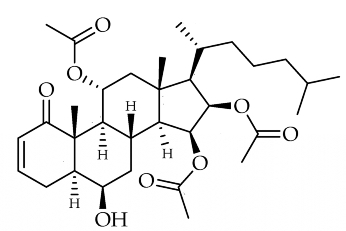
Alcyosterone
: The myeloperoxidase–H2O2–Cl system can react with the double bond in the 2nd ring of cholesterol to generate a-chlorohydrins (6-β-chloro-cholestane-( 3β,5α)-diol) and other chlorinated products (Heinecke JW et al., Biochemistry 1994, 33, 10127). Because chlorohydrins are quite stable, chlorinated sterols may prove useful as markers for lipoproteins oxidatively damaged by activated phagocytes which are known to secrete myeloperoxidase.
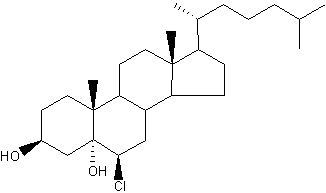
Cholesterol α-chlorohydrins
These products were also formed in LDL (Hazen SL et al., 1996) and cell membranes following exposure to HOCl or the myeloperoxidase system (Carr AC et al., Arch Biochem Biophys 1996, 332, 63). In cells, the formation of cholesterol chlorohydrins could be potentially disruptive to cell membranes as it results in cell lysis and death. They could also be potential biomarkers for oxidative damage associated with neutrophil/monocyte activation.
Phytosterols : In higher plants, the first sterols were isolated by Hesse O (1878) from the Calabar beans (Phytostigma venenosum) which coined the term “phytosterine”. This substance was later named stigmasterol (Windaus and Hault, 1906) from the plant genus. The denomination “phytosterol” was proposed in 1897 (Thoms H) for all sterols of vegetal origin. Chemically, these sterols have the same basic structure as cholesterol but differences arise from the lateral chain which is modified by the addition of one or two supernumerary carbon atoms at C-24 with either a or b chirality. The 24-alkyl group is characteristic of all phytosterols and is preserved during subsequent steroid metabolism in both fungi and plants to give hormones that regulate growth and reproduction in a manner similar to animals.
The study of the physical properties of model membranes mimicking plant plasma membranes suggests that phytosterols are more efficient than cholesterol in extending the temperature range in which membrane-associated biological processes can take place (Beck JG et al., FASEB J 2007, 21, 1714). These conclusions are in accordance with the fact that plants have to face higher temperature variations than animals.
Structural diversity, distribution, metabolism, analysis, and health-promoting uses of phytosterols have been reviewed (Moreau RA et al., Prog Lipid Res 2018, 70, 35).
More than forty phytosterols have been identified, and some plants contain up to twenty different sterols. They all have a chemical structure related to cholesterol. Most phytosterols are compounds having 28 to 30 carbon atoms and one or two carbon-carbon double bonds, typically one in the sterol nucleus and sometimes a second in the alkyl side chain.
All phytosterols were shown to derive in plants from cycloartenol (a 4,4-dimethylsterol) and in fungi (including yeasts), as in vertebrates, from lanosterol, both direct products of the cyclization of squalene.
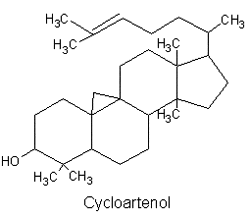
There is direct evidence to indicate that the biosynthetic pathway for phytosterol via lanosterol exists also in plant cells. This new biosynthetic pathway was designated ‘‘the lanosterol pathway” (Ohyama K et al., PNAS 2009, 106, 725).
A review has been focused on the rare group of carbon-bridged steroids, as cycloartenol, found in various natural sources such as green, yellow-green, and red algae, marine sponges, soft corals, ascidians, starfish, and other marine invertebrates (Dembitsky VM et al., Marine Drugs 2021, 19, 324). Furthermore, several of these lipids demonstrate strong antitumor activity. Phytosterols induce apoptosis, block the cell cycle, and abrogate the invasion and metastasis of cancer cells, offering a multi-manifestation treatment of cancer. Nevertheless, their clinical use is limited but preclinical and epidemiological studies have shown that phytosterols can be used as a useful adjunctive component to cancer treatments (Shahbaz M et al., Food Sci Nutr 2026, 14, e71505).
4,4-Dimethylsterols, are a special class of naturally-occurring phytosterols that possess numerous health benefits via improving the endogenous cannabinoid system. The established 4,4-dimethylsterol profile in vegetable oils was limited to few common compounds, notably cycloartenol and 24-methylene cycloartenol but many others have been detected in various plants (Zhang T et al., Food Sci Technol 2020, 124, 109163). It has been shown that 4,4-dimethylsterols have the property to regulate hepatic lipophagy via the AMPK pathway. This appears as a novel perspective on the lipid-lowering effects of phytosterols (Qian Y et al., Food Biosci 2025, 65, 105983).
Cycloartenol derivatives bearing a 9ß,19-cyclopropyl group have been shown to be frequently present in pollen of angiosperms (Villette C et al., Lipids 2015, 50, 749). Besides these compounds, three others major sterol groups have been isolated from angiosperm pollen: sterols with a double bond at C-7 (Δ7-sterols such as Δ7-campesterol), sterols with a double bond at C-5 (delta5-sterols such as campesterol) and various stanols (such as sitostanol). All these groups were unequally distributed among species. However, the distribution of sterols as free sterols or as steryl esters in pollen grains indicated that free sterols were mostly delta5-sterols and that steryl esters were predominantly 9ß,19-cyclopropyl sterols.
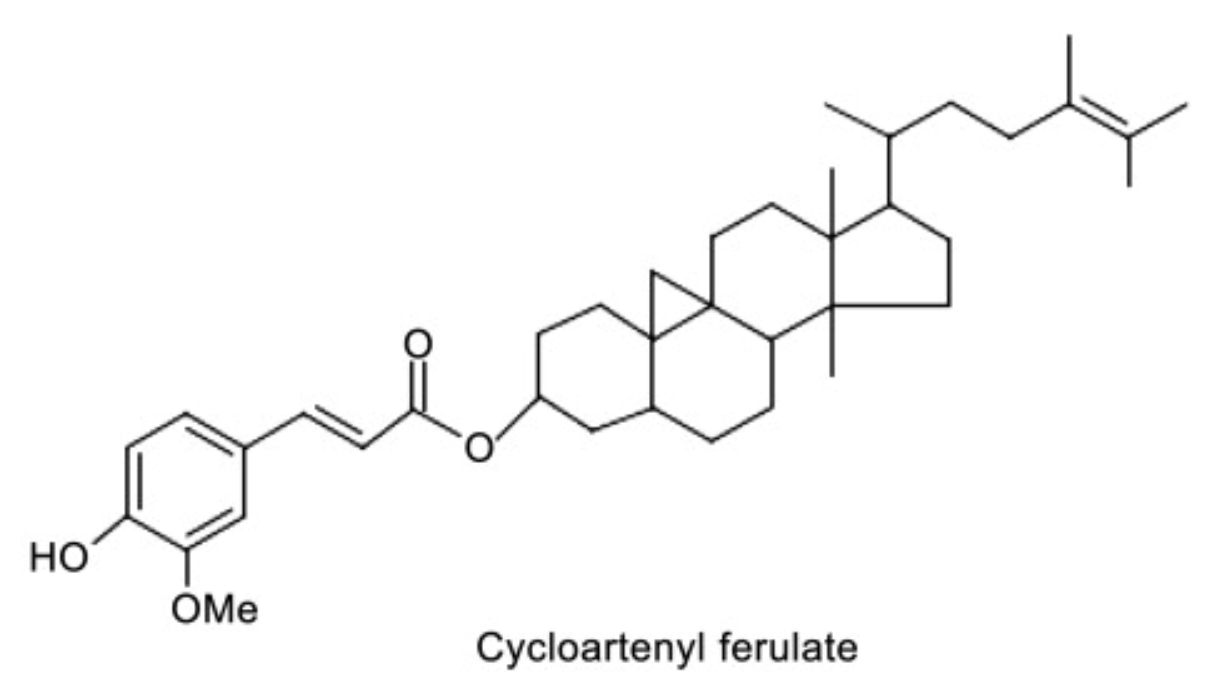
Linked with ferulic acid, several derivatives are present in γ-oryzanol, a lipid mixture extracted from rice bran (“rice oil”) (about 20% of the unsaponifiable fraction in that oil is oryzanol). Among these compounds, cycloartenyl ferulate and 24–methylene cycloartanyl ferulate are the most important. While the complete role of γ-oryzanol as a functional ingredient has not so far been thoroughly observed, several anticancer and antioxidant properties have been assigned to that lipid product. The natural bioactivity of γ-oryzanol has potential diverse applications for healthy foods (review in: Pansiri S et al., J Funct Foods 2025, 134, 107064).
Cycloastragenol is a derivative of cycloartane.(4,4,14-trimethyl-9,19-cyclo-5alpha,9beta-cholestane). It has been isolated from various legume species in the genus Astragalus (A. membranaceus) and is proposed to have telomerase activation activity (Wikipedia). The pharmaceutical properties of that plant were known 4,000 years ago iin China. Cycloastragenol appears as an exciting novel candidate for age-associated diseases (Yu Y et al., Exp Ther Med 2018, 16, 2175).
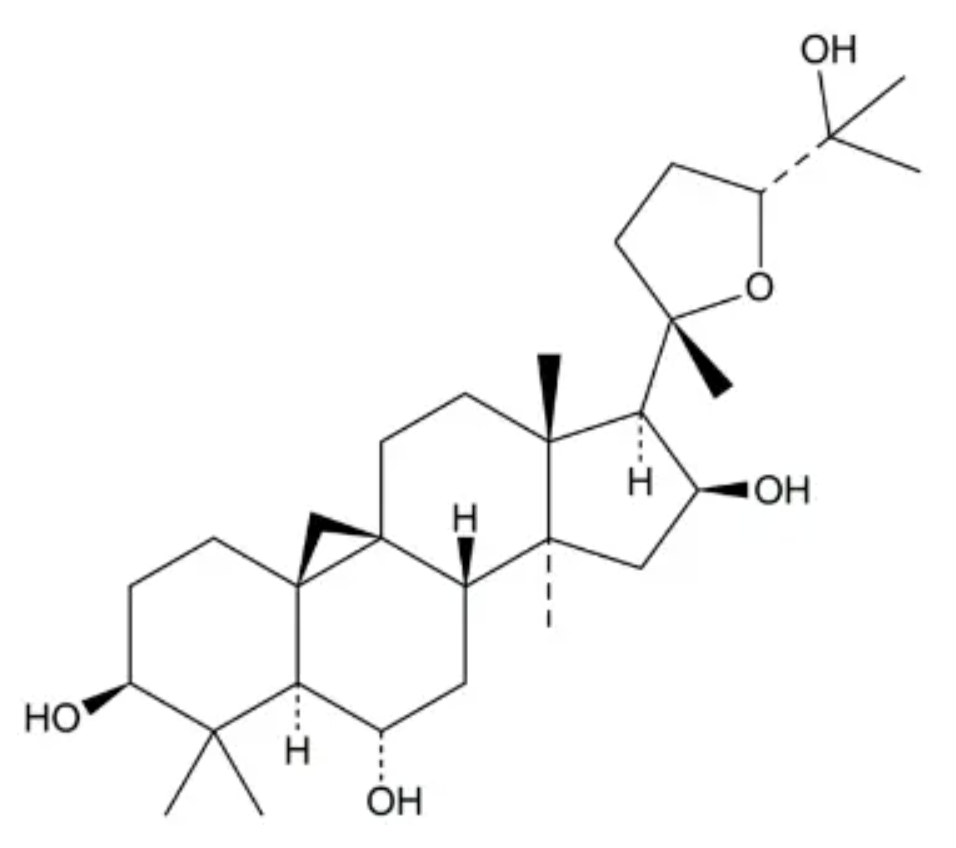 Cycloastragenol
Cycloastragenol
More than 250 different types of phytosterols have been reported in plant species. Representatives of these sterols are campesterol, β-sitosterol and stigmasterol (in soybean oil). They all belong to the group of 4-desmethyl sterols and account for 30%, 3%, and 65%, respectively, of human dietary phytosterol intake. In brown algae (Phaeophyceae) the dominant sterol is fucosterol and cholesterol is present only in low amounts.
β-Sitosterol is present in all plant lipids and is used for steroid synthesis and also in nutrition (food additive E499). It is currently studied for its potential to reduce benign prostatic hyperplasia and blood cholesterol levels by inhibiting cholesterol absorption in the intestine through inhibition of food cholesterol from gut. Clinical studies have demonstrated that consuming 2 g of plant sterols daily can effectively reduce plasma cholesterol levels by 9–14% in humans with minimal or no side effects (Law, 2000). It has been shown that that the three branches of β-sitosterol were responsible of the plasma cholesterol-lowering activity. In contrast, cholesterol analogues with a side chain of two or fewer branches did not affect plasma cholesterol (Zhu H et al., Food Chem 2024, 13 August, 140820). Studies have demonstrated that dietary fatty acids modulate rate-limiting steps in phytosterol absorption (Zhao T et al., Food Chem, online 3 October 2025, 146525).
 β-Sitosterol
β-Sitosterol
Stigmasterol, which is used for the synthesis of progesterone and vitamin D3, is known as “Wulzen factor”, a potential anti-inflammatory compound. Its action is mediated by the inhibition of several pro-inflammatory and matrix degradation mediators involved in osteoarthritis-induced cartilage degradation (Gabay O et al., Osteoathr Cartil 2010, 18, 106). It has been reported that it has cytotoxic effects on human malignant melanoma cell lines and that it exerts that property through down-regulation of reactive oxygen species and programmed cell death Ligand 1 in melanoma cells (Han NR et al., Antioxidants 2024, 13, 380).
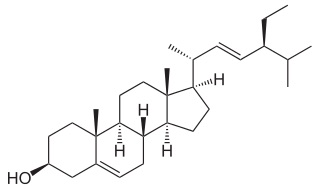 Stigmasterol
Stigmasterol
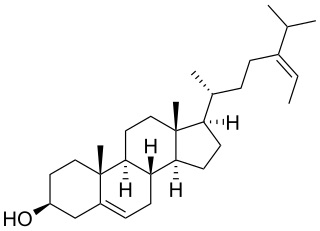
Fucosterol (24-ethylidene cholesterol) is a sterol present in algae, seaweed and diatoms. It exhibits various biological therapeutics, including anticancer, antidiabetic, antioxidant, hepatoprotective, antihyperlipidemic, antifungal, anti-histaminic, anticholinergic, anti-adipogenic, anti-photodamaging, anti-osteoporotic, blood cholesterol reducing, blood vessel thrombosis preventive and butyrylcholinesterase inhibitory activities (Abdul Q.A. et al., J Sci Food Agric 2016, 96, 1856). Fucosterol was shown to be promising against Alzheimer’s disease by targeting oxidative stress, inflammation, cholinergic deficit, amyloidogenesis, cholesterol homeostatic pathway, and signaling systems that are linked with neuronal survival (Rahman A et al., Mar Drugs 2021, 19, 167). A comprehensive review on safety and toxicity levels of fucosterol of marine algae may be consulted (Meinita MD et al., Mar Drugs 2021, 19, 545).
 Fucosterol
Fucosterol
An important sterol from yeast and ergot is the C28 compound ergosterol (mycosterol). Following the discovery of ergosterol by Tanret C (Compt rend Acad Sci Paris, 1889, 108, 98), Gerard E (Compt Rend Acad Sci France 1892, 114, 1544; idem 1898, 126, 909) observed that not only ergot, but fungi in general, contain it. Gerard was the first to recognize ergosterol in yeast. Upon irradiation, this sterol gives rise to vitamin D2 (calciferol) which, once absorbed, does not affect vitamin D status (Stephensen CB et al., J Nutr 2012, 142, 1246).
As ergosterol is a cell membrane component largely restricted to fungi, its amount in environmental matrices may be used as an index molecule for these micro-organisms in a living biomass (Barajas-Aceves M et al., J Microbiol Methods 2002, 50, 227; Charcosset JY et al., Appl Environ Microbiol 2001, 67, 2051). Three unusual C30 ergosterols, cordycepsterols A–C (1–3), have been isolated from Cordyceps militaris, a valuable edible and medicinal fungus (Chen ZA et al., J Nat Prod 2025, 88, 1499). These compounds exhibited significant inhibitory activity against nitric oxide production and could significantly suppress the secretion of tumor necrosis factor-α and interleukin-6.
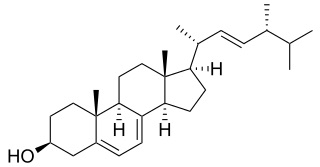
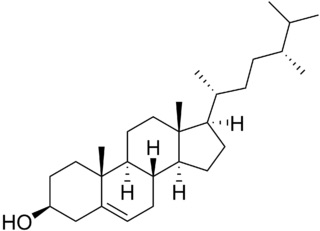
Campesterol
Phytosterols are integral components of the membrane lipid bilayer in plants. They regulate membrane fluidity and thus influence its properties, functions and structure. An increase in accumulation of phytosterols, namely campesterol, stigmasterol and β-sitosterol was observed with severity of drought stress in rice (Kumar MS et al., Plant Physiol Biochem 2015, 10, 1016). These results led to the development of an industrial product based on phytosterols which prepares various cultivated plant for a future lack of water (Elicit Plant).
An isomeric form of ergosterol, antrosterol (ergosta-7,9,22-trien-3β-ol), from the fungus Antrodia camphorata, is efficient in the protection from liver damage through its anti-inflammation capacity (Huang GJ et al., Food Chem 2012, 132, 709). This compound is likely at the origin of the use of the fungus as a traditional Chinese medicine for the treatment of drug intoxication, diarrhoea, hypertension and cancer.
A new ergosterol derivative named thalassosterol was isolated from the methanolic extract of a seagrass Thalassodendron ciliatum growing in the Red Sea (Abdelhameed RFA et al., Mar Drugs 2020, 18, 354). This derivative showed significant in vitro antiproliferative potential against the human cervical cancer cell line (HeLa) and human breast cancer (MCF-7) cell lines, with IC50 values of 8.12 and 14.24 μM, respectively.
Thalassosterol
A new cholesterol derivative, 24-methylene cholesterol, was isolated from the marine diatom Thalassiosira rotula and was found to be a potent bioactive compound against cancer. The demonstration was realized on two tumour cell models recognised as resistant to chemical treatments, the breast MCF7 and the lung A549 cell lines (Cutignano A et al., Marine Drugs 2022, 20, 595).
24-methylene cholesterol
Considerable variability in the concentration of free sterols was observed among different oils. While concentrations lower than 100 mg/100 g are found in oils from coconut, palm, olive, and avocado, concentrations between 100 and 200 mg/100 g are found in oils from peanut, safflower, soybean, borage, cottonseed, and sunflower, and concentrations between 200 and 400 mg/100 g are found in oils from sesame, canola, rapeseed, corn, and evening primrose (Phillips KM et al., J Food Comp Anal 2002, 15, 123). A unique online database (EuroFIR-BASIS) contains information on the content of 18 individual phytosterols in 91 different plants and plant based foods.
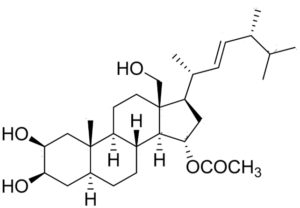
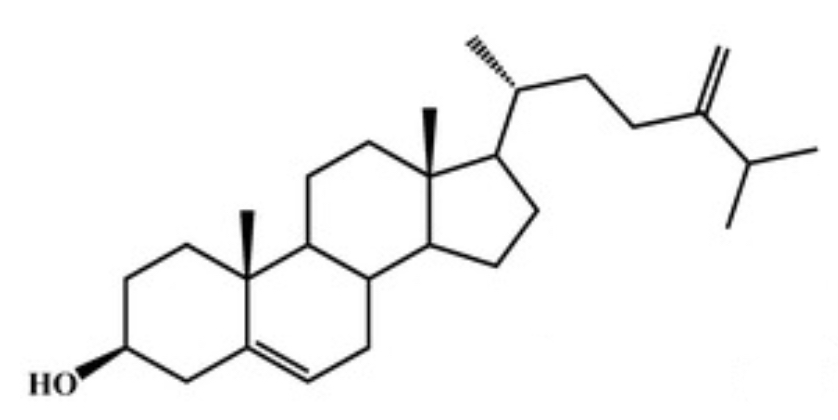
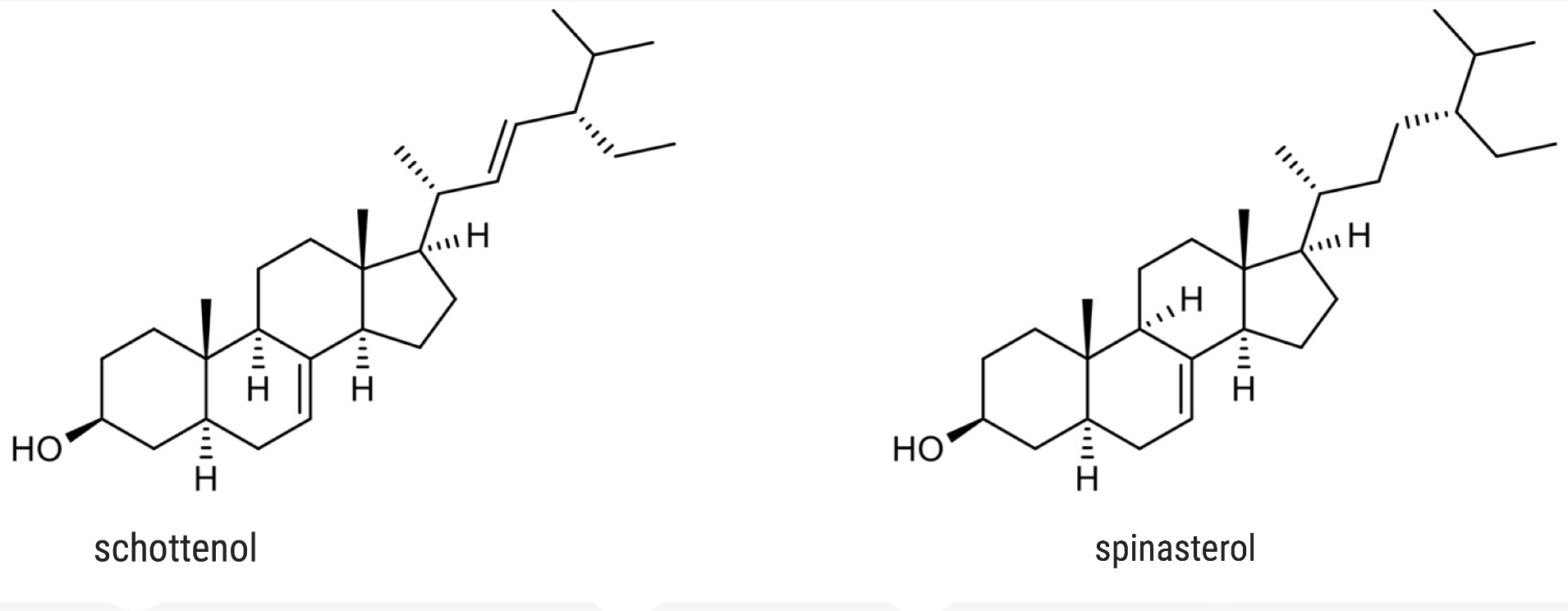
Schottenol and spinasterol are two phytosterols mainly present in argan oil and less in cactus seed oil (Essadek S et al., Pharmaceuticals 2022, 15, 465). Several investigations suggest the potential of both phytosterols to provide protection against oxidative stress (Essadek S et al., Antioxidants 2023, 12, 168). The potential role of major argan oil compounds as nuclear factor erythroid 2-related factor 2 (Nrf2) regulators and their antioxidant effects has been reviewed (El Kebbaj R et al., Antioxidants 2024, 13, 344).
Phytosterols account for a substantial portion of total dietary sterols in vertebrates but they are excluded from the body. Accumulation of other sterol than cholesterol is prevented at the level of the intestinal epithelium concurrently with a facilitation of biliary excretion of phytosterols. Phytosterols produce a wide spectrum of biological activities in animals and humans. They are considered efficient cholesterol-lowering agents (Trautwein EA et al., Eur J Lipid Sci Technol 2003, 105, 171; Ostlund RE, Lipids 2007, 42, 41). In addition, they produce a wide spectrum of therapeutic effects including anti-tumor properties. As an exemple, β-sitosterol has been shown able to improve urinary symptoms and flow measures in patients with prostatic hyperplasia, but not to reduce the size of the prostate gland (Cicero AFG et al., Arch Ital Urol Androl 2019, Oct 2;91/3). Furthermore, β-sitosterol appears efficient in suppressing prostate cancer cell proliferation, migration and invasion, but had no or minor effects on adhesion (Lu Y et al., Cancer Res 2007, 98th AACR Annual Meeting– Apr 14-18). The effects of these sterols, found in plant extracts such as Cucurbita seeds, seem to be obtained by the inhibition of 5-α-reductase. This enzyme is required to convert testosterone to dihydrotestosterone, which has a higher affinity than testosterone for androgen receptors. As a result, protein synthesis increases the volume of the prostate (Salehi B et al., Molecules 2019 2. pii: E1854).
β-Sitosterol plays a role in cancer prevention and treatment mainly by enhancing apoptosis, inducing cell cycle arrest, bidirectionally regulating oxidative stress, improving metabolic reprogramming, inhibiting invasion and metastasis, modulating immunity and inflammation, and combating drug resistance (review in : Wang H et al., Adv Notre 2023, 14, 1085).
Further data on their metabolism and potential therapeutic action can be found in a review article (Ling WH et al., Life Sci 1995, 57, 195). A review of physiologic and metabolic aspects related to these cholesterol-lowering properties may be consulted (Brufau G et al., Nutr Res 2008, 28, 217). The interest of adding sterols and stanols to human food to improve health has been discussed (Caswell H et al., Nutr Bull 2008, 33, 368). Clinical experiments have shown that only high amounts of stanols (about 9 g/day) can decrease serum β-carotene concentrations, without altering those of vitamins A, D and E (Gylling H et al., Clin Nutr 2010, 29, 112).
Saringosterol, a derivative of fucosterol, discovered in several brown algae (Phaeophyta), such as Lessonia nigrescens and Sargassum ringgoldianum, has been shown to inhibit the growth of Mycobacterium tuberculosis (Wachter GA et al., J Nat Prod 2001, 64, 1463). This phytosterol compares well with the tuberculosis drug rifampin, without appreciable toxicity against mammalian cells, and may be the source for future tuberculosis drugs.
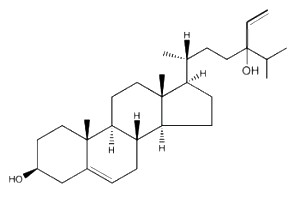
Saringosterol
A physiological study has established that saringosterol is an effective cholesterol-lowering and anti-atherogenic phytosterol. Insights concerning the underlying mechanism are given (Yan Y. et al., Marine Drugs 2021, 19).
It has been found that dietary supplementation with the seaweed Sargassum fusiforme, containing the preferential LXRβ-agonist 24(S)-saringosterol, prevented memory decline and reduced amyloid-β deposition in an Alzheimer’s disease mouse model. It was further demonstrated that 24(S)-saringosterol as a food additive is active on cognition and neuropathology in that animal model (Martens N et al., Mar Drugs 2021, 19(4), 190).
The European Commission authorized in 2004 the addition of phytosterols and phytostanols in food products with conditions of labeling including their amount per 100 g and the statement that the human consumption of more than 3 g/day should be avoided.
As cholesterol, phytosterols may undergo oxidative processes. These oxyphytosterols have been shown to have beneficial biological properties which deserve further investigations (Hovenkamp E et al., Prog Lipid Res 2008, 47, 37).
Phytostanols are a fully-saturated subgroup of phytosterols (they contain no double bonds). They are in general produced by hydrogenation of phytosterols. These saturated sterols are found in very small amounts inplant products, such as nuts, seeds and legumes but in higher amounts in tissues of a few cereal species. To improve their solubility, plant stanols are often combined with a fatty acid ester to produce plant stanol esters. The most generally found stanol is sitostanol.
Sitostanol
Stanols often occur in dinoflagellates but are not common in other marine microalgae. Hence, dinoflagellates are often the major direct source of 5 α(H)-stanols in marine sediments (Robinson N et al., Nature 1984, 308, 439).
Fully saturated sterols are also found in animals but are of bacterial origin. Thus, the 5β(H)-stanol, coprostanol, constitutes approximately 60% of the total sterols in human faeces (the usual product of cholesterol reduction outside the gut, in mammalian tissues and sediments, is 5α-cholestan-3β-ol). It has been routinely studied as a marker of (modern) sewage pollution in marine and lacustrine sediments. This has led also to archaeological research, in order to detect the presence of anciet faecal material (Bethel PH et al., J Archaeol Sci 1994, 21, 619). It has been established that the ratio of coprostanol to other sterols in an ancient sample confirmed them to be “unequivocally” human in origin.
Later, the analysis of faecal stanols in lake sediment cores offered an opportunity to reconstruct human population change associated with climate events in the Maya lowlands (Keenan B et al., Quat Sci Rev 2021, 258, 1069).
Coprostanol
After the publication of the results of animal studies (Ikeda I et al., Atherosclerosis 1978, 30, 227) showing that phytostanols are more effective than phytosterols in inhibiting cholesterol absorption and reducing serum levels, research was focusing on phytostanols. Thus, similar studies have been conducted in humans where similar observations were found. To increase bioavailibility, fat-soluble phytostanol esters were incorporated into foods high in fat. The first product marketed in 1995 was margarine, a food commonly consumed in Western countries (Cater NB, Eur Heart J 1999, 1, S36). Plant stanols inhibit the intestinal absorption of cholesterol and therefore reduce serum cholesterol concentrations in animals and humans. This inhibition is ensured by the great similarity of their physicochemical properties with those of cholesterol. Consequently, foods enriched with esterified stanols constitute real progress in the dietary management of patients with hypercholesterolemia. To generate stannous, sterols are extracted and hydrogenated. Consequently, no plant source rich in stanols is exploited due to their very low profitability.
Dietary plant stanol ester consumption was shown to improve immune function in asthma patients (Florence B et al., Am J Clin Nutr 2016, 103, 444). Later, it was shown that plant stanol consumption increased anti-COVID-19 antibody responses, independent of changes in serum cholesterol concentrations (van Brakel L et al., Am J Clin Nutr 2024, jan 24).
Although practical, the ancient distinction between zoosterols, mycosterols and phytosterols is no more used, since the same sterol may have different sources, but the appellation phytosterol is actually more frequently used.
Sterols are often isolated in the unsaponifiable fraction of any lipid extract and determined by various chromatographic procedures (HPLC or GLC).
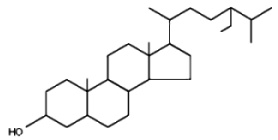
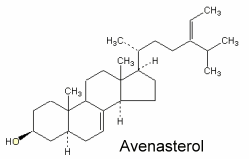
Avenasterol can be isolated from oat oil. This sterol was shown to protect specifically frying oils from oxidation owing to its ethylidene group in the side chain (White PJ et al., JAOCS 1986, 63, 525).
An extensive review on the diversity, analysis, and health-promoting uses of phytosterols and phytostanols may be consulted with interest (Moreau RA et al., Prog Lipid Res 2002, 41, 457).
It must be noticed that sterols are of widespread occurrence and have a long persistency in sediment and, thus, are found in aged geological samples (Nishimura M et al., Geochim Cosmochim Acta 1977, 41, 38).
Coprostanol, dihydrocholesterol and stigmastanol are frequently found in these sediments.
Completely saturated sterols (steranes) are derived from steroids or sterols via diagenetic degradation and saturation. They are sometimes used as biomarkers mainly for the presence of life in sedimentary organic matter and petroleum. The most abundant have 27 to 29 carbon atoms, although C30 dinosterol derivatives also occur. Thus, the most common steroids found in sediments are the regular C27-C29 steranes. Sterenes (dehydrated sterols) )are key intermediates in the conversion of oxygenated steroids into saturated steranes. A broad range of monounsaturated sterenes have been detected in immature sediments (Lu H et al., Org Geochem 2009, 40, 902). Oil bleaching introduced a maximum concentration of 5 μg/g of total sterenes into various edible oils. Deodorization is the dominant stage in sterene production resulting in a maximum total concentration of 163 μg/g. This increased concentration arises due to dehydration of the sterols and considerable isomerism of the sterenes (Colin DM et al., J High Resol Chromatogr 1994, 17, 831). Sterenes (Cholest-2-ene, cholestadiene, cholestatriene, 24-methylcholest-2-ene, 24-methylcholestadiene, 24-ethylcholest-2-ene and 24-ethylcholestadiene) have been reported in surface marine sediments in high concentrations. The formation of these compounds via microbiological processes or chemical auto-oxidation, followed by subsequent dehydration mechanisms and double bond isomerizations was postulated (Gagosian RB et al., Geochim Cosmochim Acta
1978, 42, 1091).
24-Isopropylcholestane, the hydrocarbon remains of C30 sterols produced by marine demosponges, records the presence of Metazoa in the geological record during the neoproterozoic era (1,000-542 millions years ago) (Love GD et al., Geochem Cosmochim Acta 2006, 70, A625). This sterol represents the oldest evidence for animals in the fossil record and may provide a powerful biomarker for early animal diversification (Kodner RB et al., PNAS 2008, 105, 9897). As far as known, 24-isopropylcholesterol is not synthesized by eumetazoans (cnidarians plus bilaterian animals).
Steroids containing a tertiary butyl group(s) [or tert-butyl unit(s)] are rather rare compounds that have been found in nature. A comprehensive survey of several natural steroids containing a tertiary butyl group obtained from terrestrial and marine organisms may be consulted (Dembitsky VM et al., Eur J Biomed Pharm sci 2017, 4, 32).
The first three tert-butyl steroids (neo steroids), which are called 3β-methoxy-24-methyl-lanost-9(11)-en-24-ol (1), 24ζ-methoxy-24,25-dimethyl-lanost-9(11)-en-3-one (2), and 3β,24ζ-dimethoxy-24,25-dimethyllanost-9(11)-ene (3), were isolated from Neolitsea pulchella (Lauraceae) in the early 1970s (Hui WH et al., J. Chem. Soc, 1971, 16C, 826; Chan WS, et al.J. Chem. Soc, Perkin Trans 1, 1973, 5, 490). Several others have been later isolated from plants and marine invertebrates.
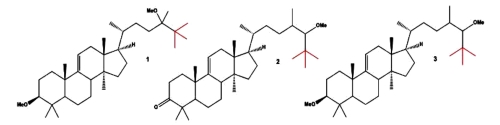
tert-butyl steroids
![]()
If sterols occur in the free state in cellular membranes in intimate association with phospholipid molecules, they are frequently found esterified to fatty acids. In animal tissues, especially in the liver, adrenals and plasma lipids (more the 70% in circulating lipoproteins), cholesterol is esterified by a variety of fatty acids and most frequently by essential fatty acids, thus forming cholesterol esters. Thus, the esterification of cholesterol with arachidonic acid gives cholesteryl arachidonate. Sterol esters are important but highly variable components of the yeast cell with values ranging from traces to 50% of the total lipids.

The chemical bonding between the sterol and the fatty acid is hydrolyzed or transesterified much more slowly than most O-acyl lipids. In animals, the esterification of free cholesterol within intestinal cells (by acyl CoA:cholesterol acyltransferase, ACAT) allows the cholesterol to be stored as a neutral lipid in cytosolic droplets and in the packing of cholesterol into lipoprotein particles for export via the plasma to liver cells. The secretions of human meibomian glands (sebaceous glands at the rim of eyelids) are particularly rich in cholesterol esters (about 30% of the lipid pool) which are characterized by a saturated or monounsaturated fatty acid moiety with C18-C34 carbon chain (Butovich IA, J Lipid Res 2009, 50, 501). Cholesteryl linoleate and cholesteryl arachidonate present in nasal fluid have been shown to contribute to the inherent antibacterial activity of that secretion (Do TQ et al., J Immunol 2008, 181, 4177). Curiously, it has been shown that the cholesteryl esters isolated from squid oil contain only saturated fatty acids (Galliani G et al., Eur J Lipid Sci Technol 2016, 118, 453).
Cholesteryl nitrolinoleate, a nitrated lipid has been detected in human blood plasma and lipoproteins (Lima ES et al., J. Lipid Res 2003, 44, 1660). Nitrated cholesterol linoleate can be considered as potential indicator of the chain-breaking antioxidant role of •NO during lipid peroxidation, as previously reported (Rubbo, H et al., Arch Biochem Biophys 1995, 324, 15). Nitrated linoleic acid has been well studied in several biological models and appears now as a potent signaling molecule. The level of cholesteryl nitrolinoleate was shown to be largely increased after macrophage activation, suggesting that lipid nitration occurs as part of the response to inflammatory stimuli (Ferreira AM et al., Biochem J 2009, 417, 223). That bioactive lipid may act as a down-regulator of inflammatory responses.
In plants, several sterol esters can be found in cell membranes and seed oils, such as ergosteryl, stigmasteryl and b-sitosteryl esters. In bryophytes (Hepaticae), cycloartenol and stigmasterol esters have been isolated (Toyota M et al., J Oleo Sci 2006, 55, 579). The relative importance of esterified sterols depends on the vegetal oil, 50-70% being found in oils from evening primrose, avocado, rapeseed, canola, corn, peanut, and sunflower, 30-50% in oils from borage, olive, sesame, coconut, and cottonseed, and less than 30% in oils from safflower, palm, and soybean. Thus, a large variation in the content and distribution of the sterol fractions between different vegetal oils can be observed (Verleyen T et al., JAOCS 2002, 79, 117). Variability reflects also differences in processing of oils and in growing season of the plant source (Phillips KM et al., J Food Comp Anal 2002, 15, 123).
In addition to variations in quantities, yeast sterol esters have been found to vary in both the sterol and fatty acid components. Fatty acids have a carbon chain from 12 up to 18 carbon atoms, saturated or having one to three double bonds. Investigations have shown than more than 20 different sterols occur in the esterified form.
In addition, cholesterol can form ester linkages with a class of secreted polypeptide signaling molecules encoded by the hedgehog gene family. These proteins function in several patterning events during metazoan development (Mann R et al., Biochim Biophys Acta 2000, 1529, 188). Observations suggest that cholesterol modification of polypeptides may be not unique to the Hedgehog proteins.
The life cycle of sterol esters, their synthesis, storage and degradation, has been reviewed (Athenstaedt K et al., Cell Mol Life Sci 2006, 63, 1355).
The presence of these esterified forms justifies a previous saponification if an estimation of the total sterol content is needed.
![]()
STERYL ALKYL ETHERS
Steryl alkyl ethers have been reported to occur only in marine sediments up to Cretaceous age. They were first reported in sediments from Walvis Bay (Boon JJ et al., Marine Chem 1979, 7, 117). Mass spectral characteristics indicate that these steryl alkyl ethers consist of C27–C29 sterols with 1-2 double bonds, that are ether-bound to C8–C9 alkyl chains. The detailed characterization of the structures of some of the dominant sedimentary steryl alkyl ethers have been reported (Schouten S et al., Org Geochem 2005, 36, 1323). Mass chromatography revealed that they are mainly composed of C27–C29 steroid moieties with one double bond and ether-bound to a C10-C12 alkyl moiety.
One of the most frequent structure (cholest-5-enyl 3β-(3-dodecanyl) ether) found in Pleistocene Atlantic sediments is shown below.
Based on their occurrence in sediments with a high diatom input, it was suggested that yet unknown diatoms should be a direct biological source.
![]()
STERYL GLYCOSIDES
Glycosylated steryl derivatives are synthesized by most plants and fungi, few bacteria and some animal cells.
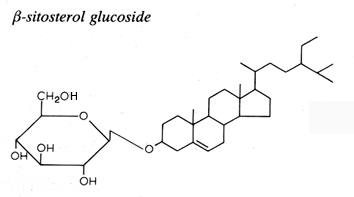
This family consists of one carbohydrate unit linked to the hydroxyl group of one sterol molecule.
The sterol moiety was determined to be composed of various sterols: campesterol, stigmasterol, sitosterol, brassicasterol, dihydrositosterol and cholesterol. The sugar moiety is composed of glucose, xylose and even arabinose (Graminae).
In bacteria, Helicobacter was shown to be particularly rich in cholesterol glucosides (up to 33% of total lipids), thus suggesting that these molecules may be important chemotaxonomic markers for these species (Haque M et al.,J Bacteriol 1995, 177, 5334).
The presence of cholesterol diglucoside was reported in a procaryote (Acholeplasma axanthum) (Mayberry WR et al., Biochim Biophys Acta 1983, 752, 434).
In plants, it has been suggested that sterol glucosides participate to the synthesis of cellulose (Peng L et al., Science 2002, 295, 147). This glycolipid is used as a substrate to produce higher homologues of the cellobioside type with beta-1,4-linked glucosyl residues. The resulting disaccharide is split off and used as primer for further elongation to cellulose.
The presence of sterol-glucosides is poorly documented in mammalian cells. Indications of the existence of glucosyl-β-D-cholesterol or 1-O-cholesteryl-β-D-glucopyranoside in mammalian cells were first provided by Kunimoto (Kunimoto S. et al., Cell Stress Chaperones 2000, 5, 3). It has been demonstrated that mammalian (mice) tissues contain glucosyl-β-D-cholesterol formed by transglucosylation through β-glucosidases using glucosyl ceramide as donor (Marques AR et al., J Lipid Res 2016, 57, 451; review in:Akiyama H et al., Biochim Biophys Acta Gen Subj 2017, 1861, 2507). It has been discovered that glucosylated cholesterol accumulates in human atherosclerotic lesions and impacts macrophage immune response (Marques ARA et al., J Lipid Res 2025, 66, 100825). These findings demonstrate that glycosidase-mediated lipid modifications may play a role in the etiology of genetic and acquired lysosomal storage disorder.
The presence of galactosylated cholesterol (β-GalChol) has been demonstrated for the first time in rat brain and its metabolism was also traced (Akiyama H et al., J Biol Chem 2020, 295, 5257). They found that β-GalChol expression depends on galactosylceramide, and developmental onset of β-GalChol biosynthesis appeared to be during myelination.
It has been demonstrated that cholesterol may be xylosylated in vitro by glucocerebrosidase, a lysosomal β-glucosidase, generating not only XylChol but notably also di-xylosyl-cholesterol and even small amounts of tri-xylosyl-cholesterol (Xyl2Chol and Xyl3Chol, respectively) (Boer DE et al., J Lipid Res 2021, 62, 100018). XylChol was detected in lysates of several types of cells cells as well as in human spleen and mouse liver. The XylChol levels are relatively low as compared with those of GlcChol and/or GalChol.
Cholesterol glucuronide was isolated from human liver (Hara A et al., Lipids 1982, 17, 515), its content being about 33 nmol/g wet tissue. The authors have isolated this compound from the acidic lipid fraction and emphasized that it cannot be distinguished readily from ganglioside GM4 by TLC. Cholesterol glucuronide is presumably synthesized in the liver and some of it enters the bloodstream (where it is present at a concentration of about 6 mg/ml), the rest being probably eliminated into the bile.
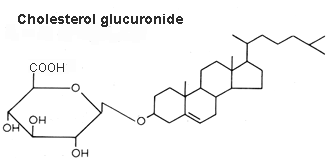
An important review of the structural diversity and occurrence of steryl glycosides, their biosynthesis, their intracellular localization and their functions my be consulted for further details (Grille S et al., Prog Lipid Res 2010, 49, 262).
![]()
These compounds are formed when a fatty acid is found acylated at the primary alcohol group of the carbohydrate unit (glucose or galactose, see figure above) in the steryl glycoside molecule (Lepage M, J Lipid Res 1964, 5, 587). Thus, 6′-palmitoyl-β-D-glucoside of b-sitosterol is the major species (51%) detected in potato tubers while 6′-linoleoyl-β-D- glucoside of β-sitosterol is predominant (47%) in soybean extracts. In these products, other fatty acids were also detected (16:1, 18:1, 18:3). More complex molecules were reported in some aquatic plants (Pistia stratiotes) where sitosterol glycosides are acylated with acetyl groups (C2′ and C4′) beside a stearyl residue (C6′) on the sugar (Della Greca M et al. Phytochemistry 1991, 30, 2422).
In a recent survey of 48 plant sources, it was shown that acylated steryl glucoside is present at concentrations from 1 to 125 mg per 100 g fresh weight in all kinds of vegetable parts (fruit, tuber, root, stem, leaf, cereals), the acylated form being 2 to 10 times more abundant that the non acylated sterol glycoside itself (Sugawara T et al., Lipids 1999, 34, 1231).
In a plant (Edgeworthia chrysantha), it was demonstrated the presence of two steryl glycosides (sitosterol glucopyranoside acylated with linoleic or linolenic acid) which have piscicidal activities (at a concentration of 100 ppm they kill Oryzias latipes within about two hours) (Hashimoto T et al. Phytochemistry 1991, 30, 2927).
![]()
SULFATED STERYL GLYCOSIDES
Several sulfated and polyhydroxylated steroid glycosides have been described in the starfish Linckia laevigata (Kicha AA et al., Chem Nat Compounds 2007, 43, 76). The most unusual chemical structure is that of linckoside, a diglycoside compound, with one glycoside moiety being a xylopyranosyl, the other a methyl xylopyranosyl.
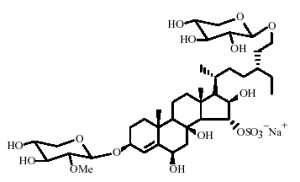
Four other homologue structures but with only one glycoside moiety have been also described.
![]()
STEROL SULFATE and SULFUR-CONTAINING STEROIDS
Sulfated steroids are found in many animals, reptiles, and humans. These compounds are also present in extracts of plants, and produced by microorganisms, fungi, and found in many marine invertebrates such as brittle star, starfish, sponges, ascidian and snails. More than 70 sterol sulfates have been described, mainly in marine invertebrates (Riccio R et al., Chem Rev 1993, 93, 1839).
These compounds have one to three sulfate groups, linked to the tetracycle core or to the lateral chain. Furthermore, sulfur-containing steroids (and terpenoids) are found in ancient earth rocks, crude oil, and other marine and aquatic sediments.
Sulfated steroids are known to present potential interest for practical and clinical medicine. Thus, they have characteristic antibacterial, antiviral properties and strong antitumor activity (review in : Pounina TA et al., Mar Drugs 2021, 19, 240).
Sulfate ester of cholesterol occurs in mammalian cells. Thus, a cholesterol-3-O-sulfate has been detected in red blood cells and mainly in skin keratinized layers where it plays a role in the desquamation process. It has been demonstrated that cholesterol sulfate fluidizes the sterol fraction of the stratum corneum lipid phase and increases its permeability (Fandrei F et al., J Lipid Res 2022, 63, 100177). Over the years, cholesterol sulfate has been found to play critical roles in various physiological processes, including not only epidermal cell adhesion but also sperm capacitation, platelet adhesion, coagulation, glucolipid metabolism, bone metabolism, gut microbiota metabolism, neurosteroid biosynthesis, T-cell receptor signaling, and immune cell migration. Pathophysiological implications and potential therapeutics of that sterol derivative have been reviewed (Yu X et al., Biomolecules 2025, 15, 646).
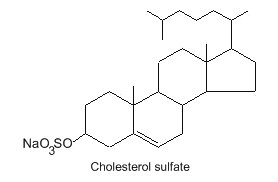
Squalamine, a condensation of cholestane 24-sulfate with spermidine in the 3β position, was discovered in shark stomach and was shown to be an efficient and broad-spectrum antibiotic (Moore KS et al., Proc Natl Acad Sci USA 1993, 90, 1354). This compound which may be a potential host-defense agent cannot be considered as a true lipid but at physiological pH, it exists as a positively charged zwitterionic molecule, soluble in both water and organic solvents. Later studies have shown that squalamine inhibits angiogenesis and endothelial cell proliferation (Hao D et al., Clin Cancer Res 2003, 9, 2465) and thus could be of value for fighting against several pathologies (cancer, macular degeneration). The presence of squalamine in lamprey white blood cells which are immune cells makes it reasonable to speculate that this molecule evolved in lower vertebrates as an immune effector (Yun SS et al., J Lipid Res 2007, 48, 2579). Trodusquemine is a similar molecule but it differs from squalamine in the polyamine moiety: being linked to C-3, which is a spermidine in squalamine and spermine for trodusquemine.
A review points to the evidence that these molecules offer promising opportunities for chronic treatments for these progressive conditions by preventing both the formation of neurotoxic oligomers and their interaction with cell membranes (Limbocker R et al., Nat Prod Rep, 2022,39, 742).
A sulfated oxysterol, a cholesterol-3-O-sulfate hydroxylated on C-25 (25HC3S; 5-cholesten-3β,25-diol 3-sulfate), has been found at high levels in the rat hepatocytes (mitochondria, nucleus) (Ren S et al., J Lipid Res 2006, 47, 1081). Later, that sulfated oxysterol has been shown to be a potent regulator of lipid metabolism in human hepatocytes (Ren S et al., Biochem Biophys Res Comm 2007, 360, 802) and in macrophages (Ma Y et al., Am J Physiol Endocrinol Metab 2008, 295, 1369), inflammatory responses and cell proliferation (Ren S et al., J Physiol Endocrinol Metab 2014, 306,E123).
Two sulfated cholesterol metabolites identified as 3β-sulfooxy-7β-hydroxy-5-cholen-24-oic acid and 3β-sulfooxy-7-oxo-5-cholen-24-oic acid were purified from the urine of a Niemann–Pick disease type C patient (Maekawa M et al., Mass Spectrometry 2016, 5, S0053). The first compound seems a promising candidate diagnostic markers for the specific diagnosis of that disease (Maekawa M et al., J Lipid Res 2019, 60, 2074-2081). More recently, 25-Hydroxycholesterol 3-sulfate was shown to be an endogenous ligand of DNA methyltransferases in hepatocytes (Wang Y et al., J Lipid Res 2021, 62, 100063).
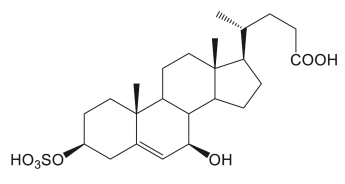 3β-sulfooxy-7β-hydroxy-5-cholen-24-oic acid
3β-sulfooxy-7β-hydroxy-5-cholen-24-oic acid
Many other sterol mono-, di-, and trisulfates have been described in invertebrates from warm waters.
Annasterol is a sterol monosulfate which was isolated from Poecillastra laminaris, a sponge living in Philippines seawater. It has potent antibacterial properties against Bacillus vulgaris (De Riccardis F et al., Tetrahedron Lett, 1992, 33, 1097).
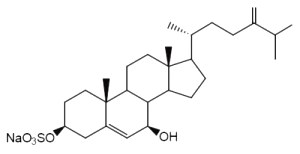
Annasterol
Marine disulfated steroids are often found in representatives of marine echinoderms, namely ophiuroids (Kicha AA et al., Marine Drugs 2022, 20, 164). Characteristic secondary metabolites of ophiuroids differ from other sulfated compounds of echinoderms in some structural peculiarities, namely in the presence of sulfoxy groups at 3α and 21 positions in 5β-, or Δ5-, and very rarely 5α-cholestane cores. Similar steroidal disulfates, containing sulfoxy groups at 3β (or 3α) and 21 positions in 5α-, or Δ5-cholestane nuclei were found in some species of the Pterasteridae family belonging to the Asteroidea class. The disulfated steroids of ophiuroids were reported to inhibit the protein tyrosine kinase, to show antiviral activity against HIV-1 and HIV-2, and to be potent antagonists of farnesoid-X-receptor. They also enhanced oxygen-dependent metabolism, increased adhesive and phagocytic properties, induced the expression of pro-inflammatory cytokines TNF-α and IL-8 in neutrophils, and enhanced the production of antibody-forming cells in the mouse spleen.
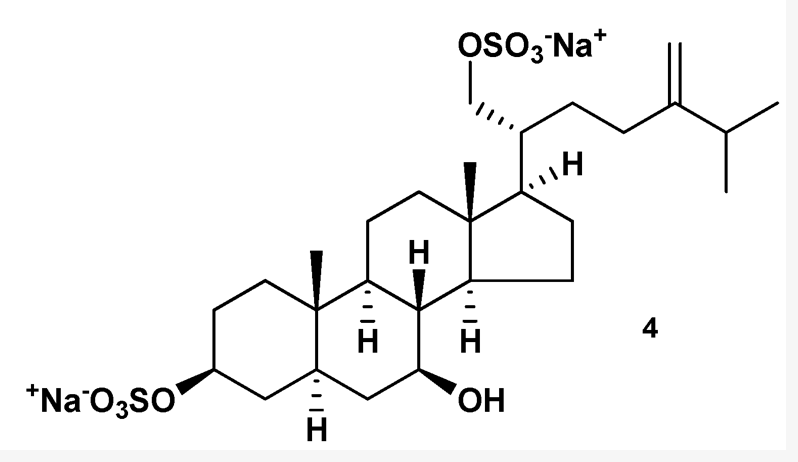 One of the disulfated ophiuroid type sterols
One of the disulfated ophiuroid type sterols
from Pteraster marsippus
Weinbersterols are sterol disulfates isolated from Petrosia weinbergi, a sponge living in Bahamas waters. One of these forms is shown below. They all have antiviral properties against the virus of cat leukemia and against VIH (Sun H H et al., Tetrahedron, 1991, 47, 1185).
.
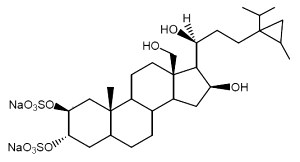
Weinbersterol A
About a dozen of sterol trisulfates have been described. They are all found in sponges and have the sulfate groups in the same position : carbon 2, 3 and 6. Some tested molecules have shown interesting antibacterial and antiviral properties (Mc Kee TC et al., J. Med. Chem., 1994, 37, 793). Several of these sulfated sterols may be found in the D’Auria’s paper (D’Auria MV et al., Chem Rev 1993, 93, 1839) (review in : Pounina TA et al., Mar Drugs 2021, 19, 240).
One of these compounds, isolated from the sponge Pseudoaxinissa digitata (Demospongia, order Axinellida) and showing anti-HIV activity is shown below.
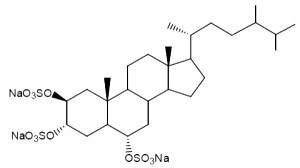
A stepwise screening approachenebled the identification of a previously uncharacterized trisulfated sterol, topsentinol L trisulfate, purified from a marine sponge Topsentia sp. collected from Papua New Guinea (El-Chaar NN et al., Mar Drugs 2021, 19, 41). It has been shown that this sterol exhibited increased effectiveness against basal-like and claudin-low breast cancer cell lines as compared with luminal and HER2+ subtypes.
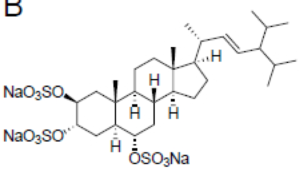
Topsentinol
Lembehsterols have similar structures, they were isolated from the marine sponge Petrosia strongylata. These sterols showed inhibitory activity against thymidine phosphorylase, which is an enzyme related to angiogenesis in solid tumors (Aoki S et al., Chem Pharm Bull 2002, 50, 827).
These molecules are currently used as models for chemists who are trying to increase their antiviral potency in modifying molecule structures.
Epithio Steroids
Sulfur-containing steroids (and triterpenoids) have been shown to be present in marine invertebrates, sediments, crude oil, and other sources (review in : Pounina TA et al., Mar Drugs 2021, 19, 240).,
Among them, semi-synthetic and synthetic epithio steroids are sulfur-containing bioactive lipids, they were not found in nature. Epithio steroids have been reported to possess a variety of cytotoxic activities, and they are widely used as anticancer agents. They form an important group of substances which show some promising biological activities.
Steroids containing an epithio group in positions 2 and 3 belong to anabolic steroids and are widely known and used in sports medicine and are of great interest for the pharmacology of sports and other aspects of medicine such as the treatment for breast cancer (review in : Pounina TA et al., Mar Drugs 2021, 19, 240).
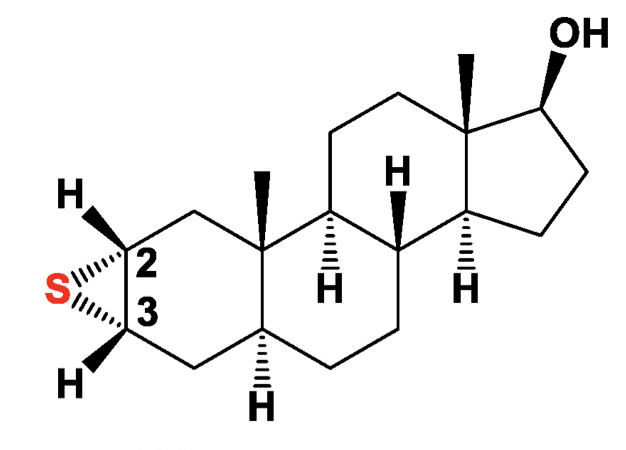
One of epithio steroids: Epitiostanol
DISPERSIVE LIQUID-LIQUID MICROEXTRACTION
Lire la suiteDevenez membre et participez au développement de la Lipidomique au XXIème siècle.
S'inscrire