
They have 20 carbon atoms and are derived from geranylgeraniol pyrophosphate.
They are of fungal or plant origin and are found in resins, gummy exudates, and in the resinous high-boiling fractions remaining after distillation of essential oils. However, unequivocal evidence was provided for de novo geranylgeraniol biosynthesis in mammals (Shidoji Y et al., J Lipid Res 2019, 60, 579).
The rosin remaining after distilling pine turpentine, for instance, is rich in diterpenoids. In ancient times, conifer exudates were used for caulking boats and waterproofing ropes. Resin secretion is also recognized to be part of the resistance mechanism conifers employ against bark beetles and their associated pathogenic fungi. Diterpenoid groups that are physiologically active include: vitamin A activity (retinol), phytohormones that regulate plant growth and germination, e.g. gibberellin, fungal hormones that stimulate the switch from asexual to sexual reproduction, e.g. trisporic acid; disease resistance agents (phytoalexins), e.g. casbene and podocarpic acid, the anticancer drug, taxol, from the bark of the yew tree, the cancer promoter, phorbol, and natural cannabinoids.
The diterpenes have exceptionally open chain, as found in geranylgeraniol or phytol which forms a part of chlorophyll and the side chain of vitamin E and vitamin K, and crocetin which is a diacid diterpenoid and the lipid part of the crocins, glycosylated derivatives present in saffron.
Nitidane was identified in the epicuticular lipid of the Springtail (Collembola) Heteromurus nitidus, representing a novel class of epicuticular lipids (Möllerke A et al., J Nat Prod 2024, 87, 1454). It is an irregular terpene consisting of seven isoprene units, made up of a diterpene core that is modified by a geranyl moiety that is itself prenylated.
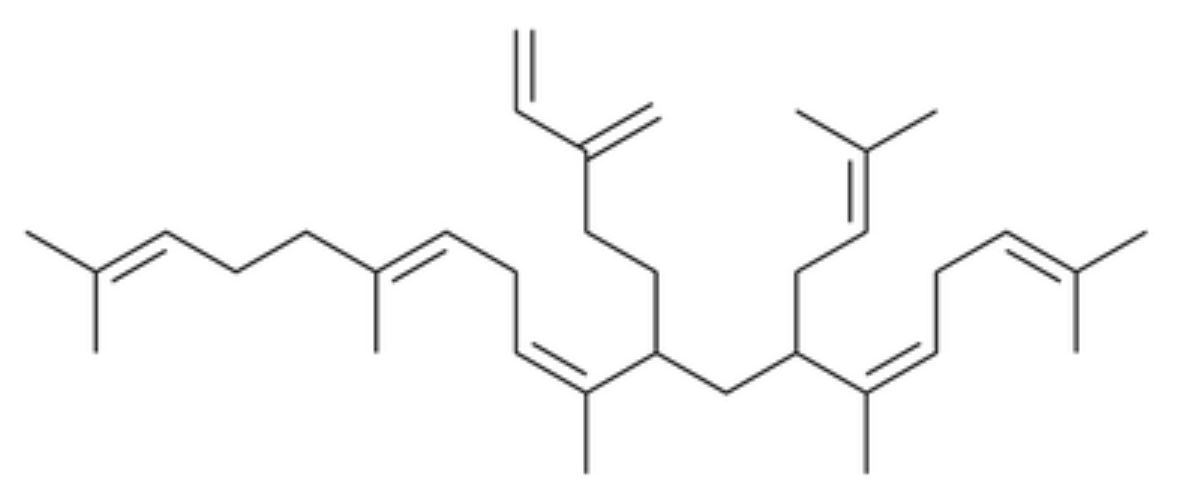 Nitidane
Nitidane
Examples of the most common diterpenes are given below :
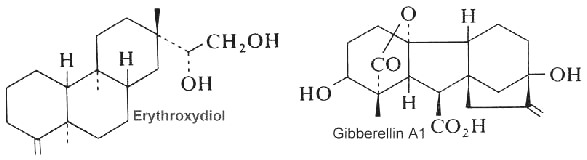
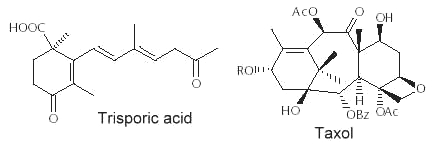
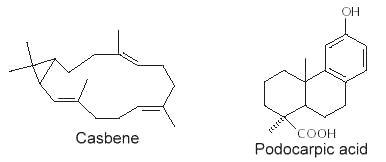
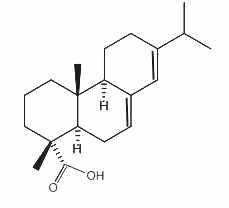
Abietic acid
Levopimaric acid, an isomeric form of abietic acid, is an abietane-type of diterpene resin acid. It is a major constituent of pine oleoresin. with various biological activities, such as antibacterial, cardiovascular and antioxidant. Its production by conifer species is an important component of the defense response against insect attack and fungal pathogen infection (Wikipedia).
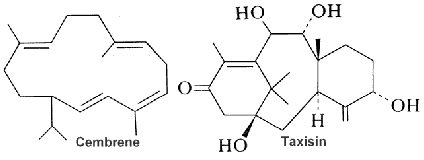
Cembrene (the simplest form of the cembranoid family) is a type of macrocyclic diterpene consisting of four isoprene units bonded ‘head to tail’. It was firstly found in the oleoresin secreted from the trunk of a pine tree. They are mainly found in plants of the Nicotiana and Pinus genera, as well as in soft coral and other marine organisms. Over 30 distinct types of these diterpene carbon skeletons have been isolated from soft corals, cembranoids being the most prevalent and representative. Structurally, cembranoids are characterized by a 14-membered carbon ring and considered to be formed by a one-step cyclization reaction involving C-1 and C-14 of geranylgeranyl pyrophosphate (Zhang N et al., Phytochemistry 2023, 212, 113703). Several cembranoids have been isolated from the Soft Coral Sarcophyton ehrenbergi (Wu MJ et al., Mar Drugs 2025, 23, 170), some of them having antibacterial propertires.
Cembranes are the most widely occurring diterpenes in Nature, they are common metabolites of several gorgonian genera (Yang QB et al., Mar. Drugs 2023, 21(1), 30).
All cembranoids feature a 14-carbon macrocyclic skeleton. About 90 naturally occurring cembranoids have been isolated from tobacco. The chemical structures, biosynthesis, and bioactivities of tobacco cembranoids have been reviewed (Yan N. et al. Indust Crops Prod 2016, 83, 66). Tobacco cembranoids are known to display antimicrobial, anti-tumour and neuroprotective properties, which make them excellent templates for the future development of drugs to treat AIDS, cancer and neurodegenerative diseases. A review discusses and summarises their antimicrobial, insecticidal, cytotoxic and neuroprotective activities (Yan N et al., Biomolecules 2019, 9: 30).
There is a considerable number of publications in the field of cembranoids of Boswellia species. The chemistry (isolation and chemical structures as well as synthetic studies), biogenesis and bioactivity of the reported cembranoids has been reviewed (Al-Harrasi A et al., Phytochemistry 2021, 191, 112897).
Abietic acid is an irritant compound present in pine wood and resin. It is the most abundant compound present in rosin, the solid fraction of the oleoresin of coniferous trees. It is mainly used to make lacquers and varnishes and metal resinates. These resinates are produced in reacting abietic acid (or a similar compound) with a metal salt (gold, indium, nickel, palladium, platinum, silver …) and used in a wide variety of applications where high purity metals in organic solution form is needed (gravure printing inks, vitrifiable colors, antifouling agent, dryers for paints and varnishes …).
Carnosic acid is a natural benzenediol abietane diterpene found in rosemary (Rosmarinus officinalis) and common sage (Salvia officinalis). Dried leaves of rosemary and sage contain 1.5 to 2.5% carnosic acid. With carnosol, a derivative of the acid, they are used as antioxidant preservatives in food and nonfood products (E392). Carnosol is an effective antioxidant that has medicinal properties by acting as an anti-inflammatory and pancreatic lipase inhibitor. It is also the subject of studies concerning anti-cancer properties.
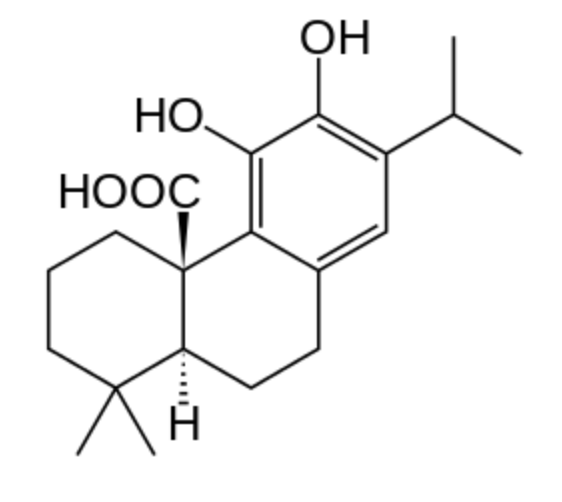
Carnosic acid
Communic acids are a group of diterpenes with labdane skeletons found in many plant species, mainly conifers, predominating in the genus Juniperus (fam. Cupresaceae). They are mainly founded in leaves, fruits, and bark. Five communic acids have been described to date, they differ in the location of the double bonds and the orientation of the carboxyl group. Trans-communic acid is the most common (Barrero AF et al., Molecules 2012, 17, 1448).
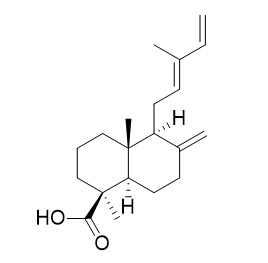 Trans-communic acid
Trans-communic acid
It was abundtly demonstratd that they have different biological activities (anti- bacterial, antitumoral, hypolipidemic, relaxing smooth muscle, etc.).
Among the various compounds of Korean red pine (Pinus densiflora) needle, 4-epi-trans-communic acid was isolated and shown to inhibit angiotensin converting enzyme (ACE) and induce p-Akt in human umbilical vein endothelial cells (Park J et al., J Med Food 2021, 24, No. 1). These results demonstrate that this isomer may be at the origin of other compounds effective for preventing hypertension.
Clerodane diterpenoids are a large group of secondary metabolites that have been isolated from several hundreds of different plant species, as well as fungi, bacteria and marine sponges. In plants, they are mainly found in Scutellaria, Croton, Ajuga, Teucrium, and Salvia (Li R et al., Nat Prod Rep 2016, 33, 1166). These diterpenoids have attracted interest in recent years due to their notable biological activities, particularly insect antifeedant properties. Diverse pharmacological activities, including antiinflammatory, antitumor, antifeedant, and neuroprotective effects have also been reported.
The distribution, chemotaxonomic significance, chemical structures, and biological activities of major clerodane diterpenes are summarized in a review (Li R et al., Nat Prod Rep. 2016, 33, 1166).
As an example, salvinorin A was the first nonnitrogenous diterpenoid with psychoactive properties, it that was first isolated from the leaves of Salvia divinorum in 1982 and has been used as a legal hallucinogen in the United States for years.
 Salvinorin A
Salvinorin A
Clerodin was originally isolated from Clerodendrum infortunatum (Lamiaceae) and later from Ajuga bracteosa. It is a potent pesticide activity due to its insect antifeedant activity.
 Clerodin
Clerodin
Caulerpol appears to be found in most species of the alga of the genus Caulerpa, usually as the dominant constituent of the crude extract. The isolation of caulerpol from Caulerpa brownii was of particular importance as it was the first compound with a retinol carbon skeleton (related to vitamin A) isolated from a plant source and was later shown to be easily synthesized from (S)-(−)-α-cyclogeraniol (Masaki Y et al., Chem Lett 1978, 1203).
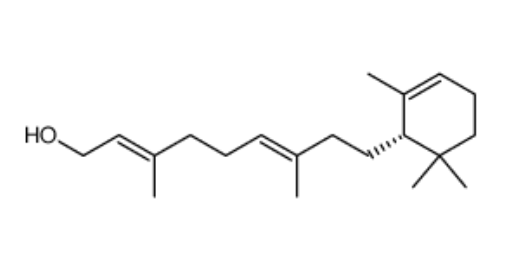 Caulerpol
Caulerpol
A series of meroterpenoids isolated from the brown alga Cystoseira usneoides have been shown to exhibit anti-inflammatory and antioxidant activities. Among those, amentadione, a meroditerpenoid, showed radical-scavenging activity and demonstrated a significant role in reducing the production of TNFα in LPS-stimulated THP-1 human macrophages (De los Reyes C et al., J Nat Prod 2016, 79, 395). Further research have shown that is could be also an efficient osteoarthritis protective agent (Araujo N et al., Marine Drugs 2010, 18, E624).
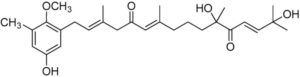
Amantadione
Sargahydroquinoic acid from Sargassum macrocarpum has been shown to attenuate TNF-α and UV-induced skin aging in human dermal fibroblasts (Cao L et al., Algal Res. 2024, 78, 103410). It was also demonstrated that these compounds have a cyclooxygenase-2 inhibitor, attenuating inflammatory responses by regulating NF-κB Inactivation and Nrf2 activation in lipopolysaccharide-stimulated cells (Young EJ et al., Inflammation 2021, 44, 2120).
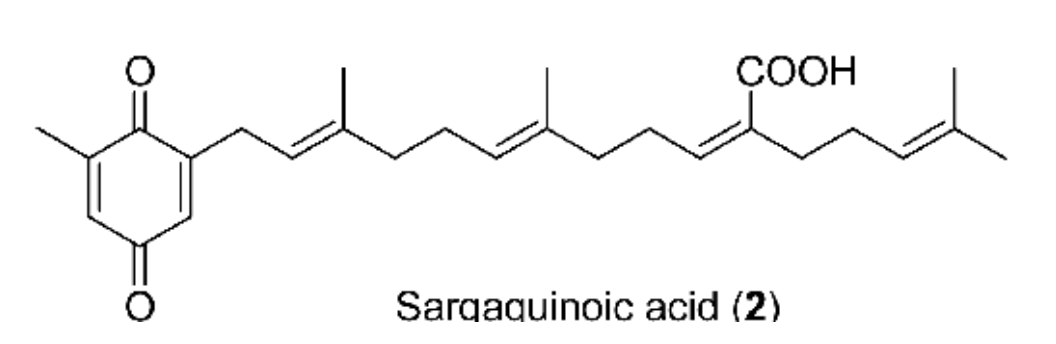
Several meroterpenoids with anticancer activity against human colorectal cancer cells have been isolated from marine sponge Hyrtios sp. (Wang J et al., Mar. Drugs 2024, 22(4), 183).
The term “meroterpenoid” was used to describe natural products bearing a mixed terpenoid biogenesis. They are a class of natural products derived from hybrid polyketide or non-polyketide and terpenoid biosynthesis. Interestingly, meroterpenoids are mostly reported from fungi and marine organisms while only a limited number of meroterpenoids were obtained from plants. Most of them possess antimicrobial, cytotoxic, antioxidant, anti-inflammatory, antiviral, enzyme inhibitory, and immunosupressive effects (Nazir M et al., Biomolecules. 2021, 11(7), 957).
The chemical study of the alga Rugulopteryx okamurae of the Family Dictyotaceae has led to the isolation of several original diterpenoids (Cuevas B et al., Marine Drugs 2021, 19, 677). Among them, rugukadiol A exhibits an unprecedented diterpenoid skeleton featuring a bridged tricyclic undecane system. It significantly inhibited the production of the inflammatory mediator NO in LPS-stimulated microglial cells and strongly inhibited the expression of Nos2 and the pro-inflammatory cytokine Il1b in two immune cell lines.
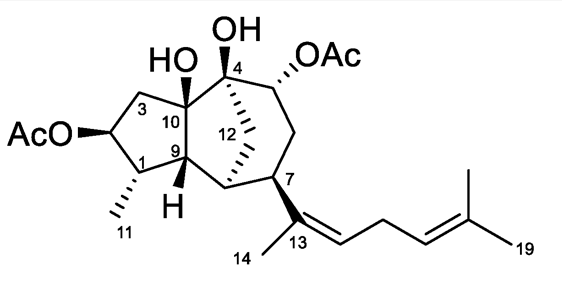
Rugukadiol 1
Halogenated tricyclic diterpenes have been extracted from the red alga Sphaerococcus coronopifolius collected from the coastline of the Ionian Sea in Greece (Smyrniotopoulos V et al., Mar Drugs 2020, 18, 29). Among them, one, a methoxy derivative of hydroxy-dihydro-sphaerococcenol, was shown to possess a potent in vitro antitumor activity against one murine and five human cancer cell lines with mean IC50 values 15 and 16 μM, respectively.
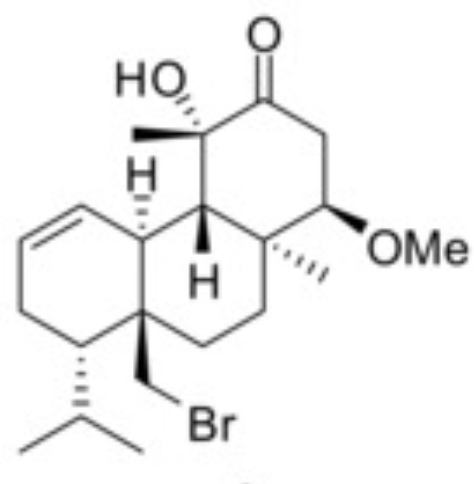
Methoxy-hydroxy-dihydro-sphaerococcenol
Some bromoditerpenes from the same seaweed were more recently determined to be potential cytotoxic agents and proteasome inhibitors (Alves C et al., Marine Drugs 202, 20, 652).
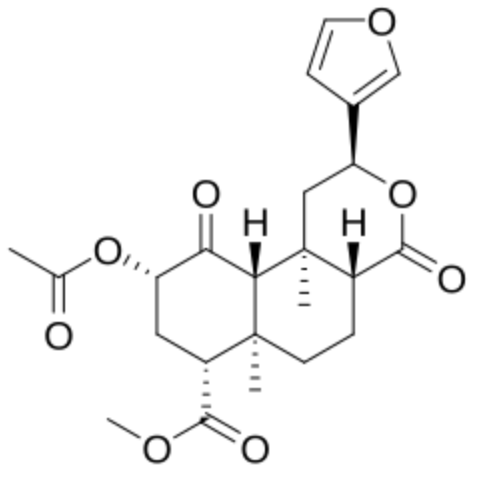
Tanshinones are abietane diterpenes, isolated mainly from Salvia miltiorrhiza (Lamiaceae), a plant largely used in traditional Chinese medicine for the treatment of cardiovascular and inflammatory diseases. Among them, tanshinone I is an apoptose inductor and displays several anticancerous biological properties (Y. Dong et al., Nat Prod Rep 2011, 28, 529). Several analogues have been synthesized for clinical trials.
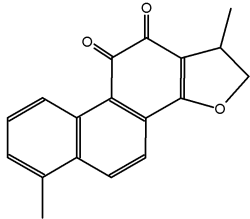
Tanshinone I
Gracilins
Gracilins are diterpenoid compounds derived from Spongionella gracilis, a marine sponge. It has been reported that these diterpenoid compounds exert anti-inflammatory properties as inhibitors of phospholipase A2 and that they exert neuroprotective and antioxidant properties (review in : Kabir MT et al., Mar Drugs 2021, 19, 251).
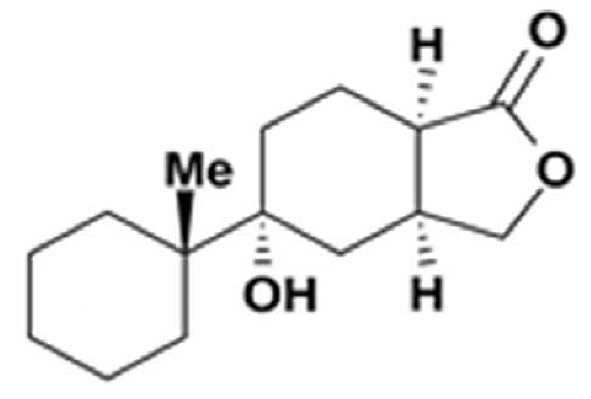
One of the Gracilins
Steviol is the aglycone of stevia‘s sweet glycosides, one of them being formed by replacing one hydrogen atom (bottom) with glucose via an ester link, and another hydrogen atom (top) with a disaccharide (glucose and rhamnose). The steviol glycosides are responsible for the sweet taste of the leaves of the stevia plant (Stevia rebaudiana, Asteraceae). These compounds are 40 to 300 times sweeter than sucrose. They are developed to be used in sweet drinks.
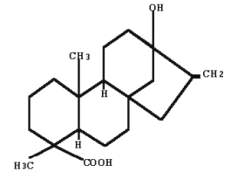
Steviol
Retene is present in tars obtained by distillation of resinous wood, it is an important pollutant eliminated by the paper factories. This diterpene is present in geological sediment where it is formed by diagenesis from abietic acid, several intermediates having been recognized (Wakeham SG et al., Geochem Cosmochim Acta 1978, 42, 289). Thus, with cadalene (sesquiterpene), retene, a diterpenoid dehydrogenation product, is used in paleobotanic to estimate the importance of ancient pine forests.
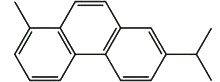
Retene
Gibberellins are a family of compounds, over 130 members exist whose structures and occurrence can be found on the web. The most important in plants is gibberellin A1 which is responsible for stem elongation. The most widely available compound is gibberellic acid (one double bond in the right cycle). Among the physiological properties, gibberellins are involved in stem growth, seed germination and fruit setting and growth.
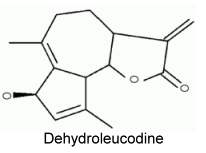
Dehydroleucodine was isolated from Artemisia douglasiana, a popular medicine in Argentina and was shown to have several physiological and therapeutic properties : anti-proliferative activity in G2 phase (Lopez ME et al., Protoplasma 2002, 219, 82), cytoprotective agent for gastric ulcers and a general antioxidant.
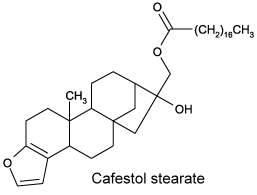
Cafestol and kahweol are present in high concentrations (up to 18% diterpene esters) in the oil derived from coffee beans. The only difference between cafestol and kahweol is an extra double bond present in the second cycle of kahweol. These diterpenes are esterified with one fatty acid (C14 to C24), palmitic and linoleic acids being the major esterified fatty acids (Folstar P et al., Lebensm Wiss Technol 1975, 8, 286). Terpenes were also shown to induce an elevated plasma cholesterol content in human (Boekschoten MV et al Nutr J 2003, 2, 8). It has been demonstrated that that these coffee diterpenes are broad-spectrum defence compounds that protect not only the seed on the mother plant and in the soil, but also the seedling after germination (Antoine G et al., Plant Physiol Biochem 2023, 194, 627).
Phorbol is a diterpene isolated in 1934 from croton oil (seeds of Croton tiglium). Various fatty acid esters of phorbol have important biological properties, the most notable of which is the capacity to act as tumor promoters through activation of protein kinase C as they mimic diacylglycerols. The most common phorbol ester is phorbol-12-myristate-13-acetate (PMA), which is used as a research tool in models of carcinogenesis.
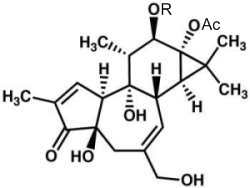
Phorbol
R : myristic acid (C14:0), Ac : acetate group
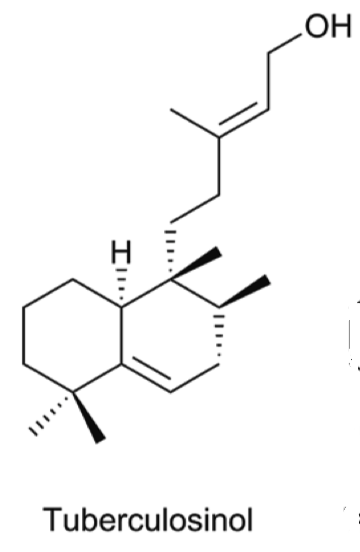
Tuberculosinol, and some analogues can be considered as virulence factors in Mycobacterium tuberculosis, a microorganism that causes tuberculosis illness, a major source of morbidity and mortality worldwide. These compounds could be targets in a new route to anti-infective therapy. They are being evaluated as biomarkers for tuberculosis (Roncero AM et al., Nat Prod Rep 2018, 35, 955).
The manool diterpene, found in abundance in Salvia officinalis L., has been shown to display a selective cytotoxic effect against murine melanoma cells (Nicolella HD et al., J Nat Prod 2022, 85, 2, 426) and a protective activity against the induction of DNA damage in human hepatocarcinoma cells (HepG2) (Nicolella H D et al., Food Chem. Toxicol. 2014, 72, 8). Salvia officinalis is a perennial woody subshrub native to the Mediterranean region that is commonly used as a condiment and as an anti-inflammatory, antioxidant and antimicrobial agent due to its biological activities.
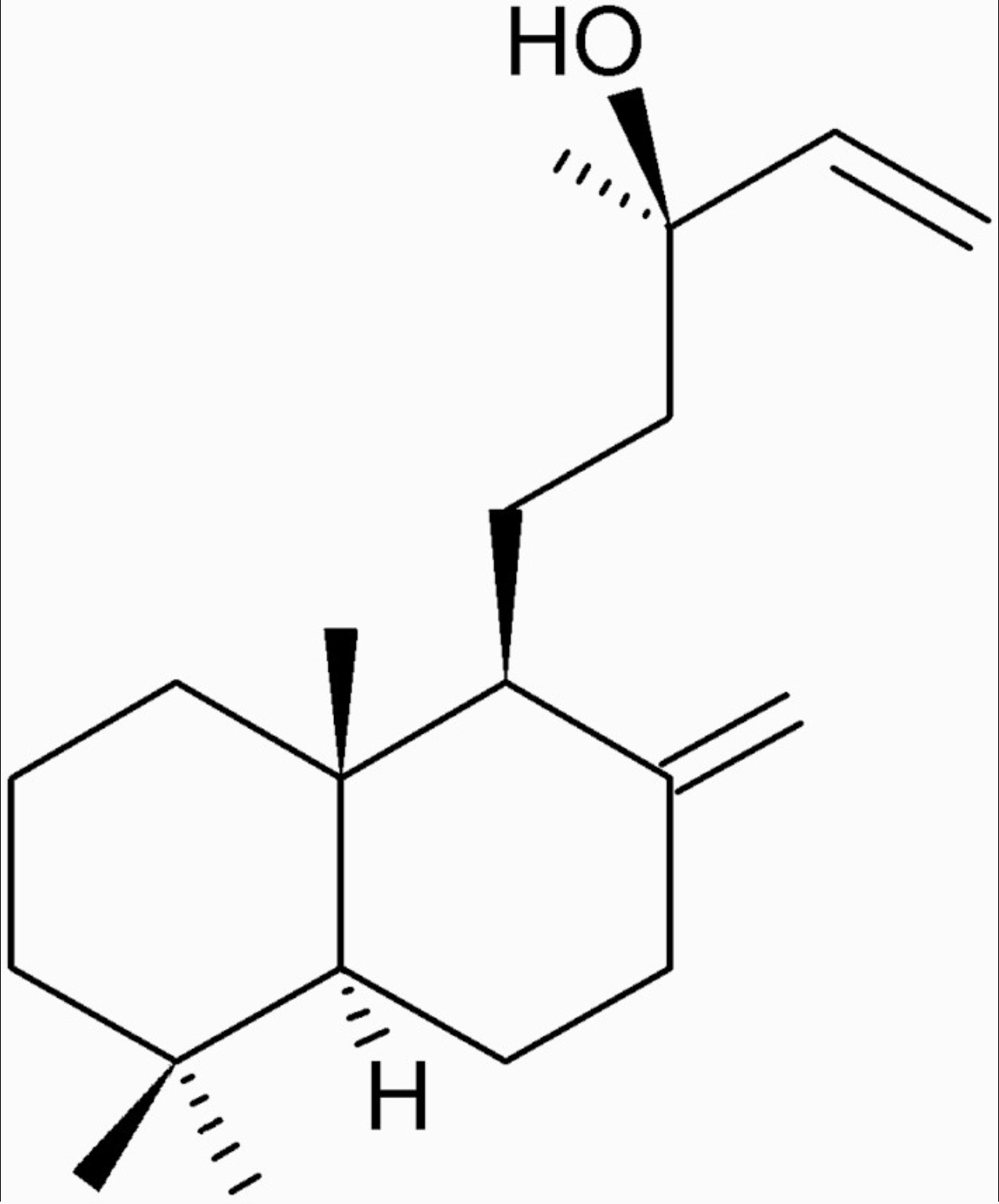 Manool
Manool
Cannabinoids are a group of diterpenes present in Cannabis (Cannabis sativa L). All these substances are structurally related to tetrahydrocannabinol (THC) and are able to bind to specific cannabinoid receptors.
Tetrahydrocannabinol, also known as Δ9-THC or Δ9-tetrahydrocannabinol, is the main psychoactive substance found in the plant. It was isolated by Mechoulam R et al. in 1964 (review in Mechoulam Ret al., Chem Phys Lipids 2000, 108, 1).
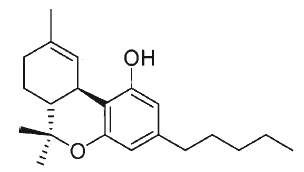
Δ9-Tetrahydrocannabinol (THC)
Research is expanding concerning some of the 120 minor cannabinoids present in the Cannabis extracts (phytocannabinoids). Cannabichromene and cannabigenol are the most studied despite they are less potent cannabinoids that THC. Research began with claims that they could promote sleeps, as stress and anxiety often lead to insomnia (Kolobaric A et al., Pharmaceuticals 2024, 17, 977).
Cannabigerol is a non-psychoactive cannabinoid, it is the decarboxylated form of cannabigerolic acid, the parent molecule from which other cannabinoids are biosynthesized. Its pharmacodynamics are complex. It is a relatively weak agonist of the cannabinoid receptors, Conversely, it is a much more potent agonist of the α2-adrenergic receptor, antagonist of the serotonin 5-HT1A receptor, and antagonist of the transient receptor potential channel TRPM8 (Wikipedia). Although cannabigerol is sold as a dietary supplement, its effects and safety for human consumption are poorly known. In basic research models such as mice or rats, cannabigerol has demonstrated anticancer properties, particularly against breast cancer, anti-inflammatory effects on murine colitis and on inflammatory bowel disease (Zagožen M et al., Acta Pharm
2020, 71, 355).
 Cannabigerol
Cannabigerol
Cannabichromene, also called cannabichrome, bears structural similarity to the other natural cannabinoids exhibits. Its anti-inflammatory properties in vitro may, theoretically, contribute to Cannabis analgesic effects (Wikipedia). It has shown anti-inflammatory effects and reduced hypermobility in the gut and has displayed potential in vitro effect on adult neural stem progenitor cells. It exerts also modest analgesic properties in rodents, as well as anti-fungal, anti-bacterial, pro-apoptotic, and anti-proliferative effects in tumor cells (Zagožen M et al., Acta Pharm 2020, 71, 355). .
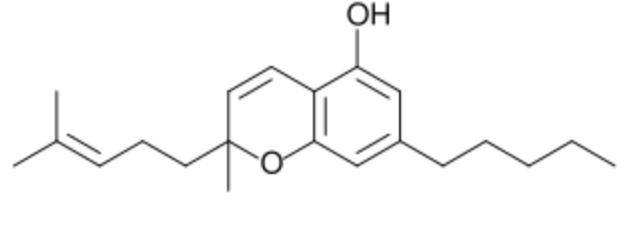 Cannabichromene
Cannabichromene
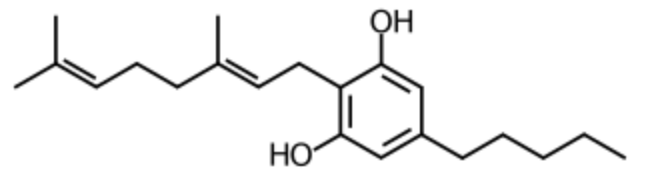
Cannabigerolic acid is the acidic form of cannabigerol. It is a dihydroxybenzoic acid in which the hydrogen at position 3 is substituted by a geranyl group (Wikipedia). It is a biosynthetic precursor to THCl which is the principal psychoactive constituent of the Cannabis plant. A 2022 pre-clinical study found that this compound could prevent infection by SARS-CoV-2 (van Breemen RB et al., J Nat Prod 2022, 85, 176).
 Cannabigerolic acid
Cannabigerolic acid
Some diterpènes (Kanakugiol, Kingidiol) present in various plants have been shown to disrupt the receptor complex of insect juvenile hormone (Lee SH et al., PNAS 2015, 112, 1733). These results indicate that newly discovered compounds could be used as the starting material for development of novel insecticides.
Andrographolide is a diterpene of the labdane family isolated from the stem and leaves of Andrographis paniculata (Acanthaceae, native to India and Sri Lanka). It has been studied for its effects on cell signaling, immunomodulation, and stroke. A study has shown that A. paniculata plant extract has anti-diabetic properties, in accordance with its local use for that disease. Andrographolide has also the property to help recover the cognitive decline in a rodent model of in a natural model of Alzheimer’s disease (Rivera DS et al., Neurobiol Aging 2016, 46, 204). These important results suggest that Andrographolide could be used in human as a potential therapy for Alzheimer’s disease. In addition, andrographolide exhibited beneficial effects on diseases related to diarrhea and intestinal inflammation (Wang P et al., J Funct Foods 2024, 123, 106568).
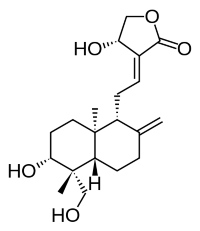
Andrographolide
Erinacines are natural substances isolated from the edible medicinal fungus Hericium erinaceus which belong to the group of cyathin diterpenoids (erinacines A-K, P, and Q) and are subjects of pharmacological research. Erinacine A is the main representative of this group, it has an enhancing effect on nerve growth factor synthesis in vitro (Shimbo M et al., Nutr Res 2005, 25, 617). Erinacine A found in the fungus mycelia has been confirmed to have pharmacological effects on the central nervous system, i.e., long-term prevention of stroke, Parkinson’s disease, Alzheimer’s disease, depression, and nerve-related diseases (Bailly C et al., Pharmacol Res 2020, 159, 104953).
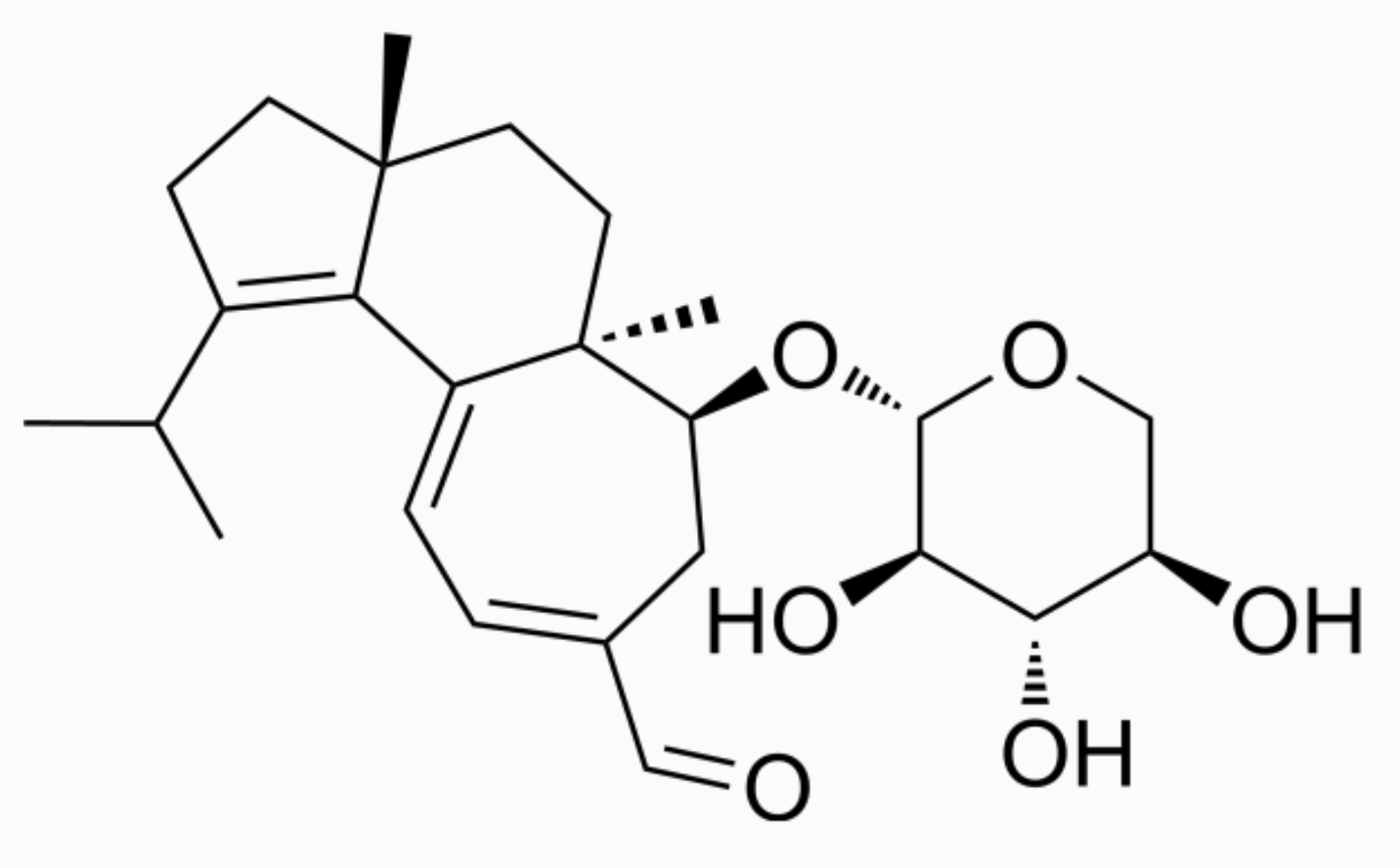 Erinacine A
Erinacine A
Furanoditerpenoids, a special group of diterpenoids, have a structure including one or more furan rings in core skeleton. Due to the existence of that furan ring, they always show significant biological activities, which have been intensively investigated in past several decades (Bao H et al., Phytochem Rev 2017, 16, 235). The largest number of furanoditerpenoids have been described in the families Euphorbiaceae, Fabaceae
and Lamiaceae. Most of these plants are widely used in traditional medicines, and furanoditerpenoids have therefore been disclosed with a wide range of bioactivities including anti-cancer, anti-inflammation and anti-microorganism. Up to now more than 440 furanoditerpenoids have been isolated and identified from plants and microorganisms. Only two examples of these compounds are given below:
Salvinorin A, isolated from Salvia divinorum, has been proved to produce psychoactive experiences in human, it has been used as an analgesic.
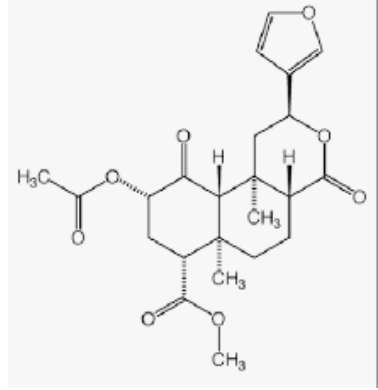
Salvinorin A
Kahweol, isolated from the beans of Coffea arabica, is a potent inhibitor of osteoclast differentiation, it has also anti-inflammatory and anti-angiogenic properties.
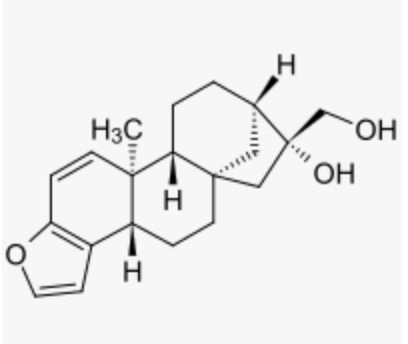
Kahweol
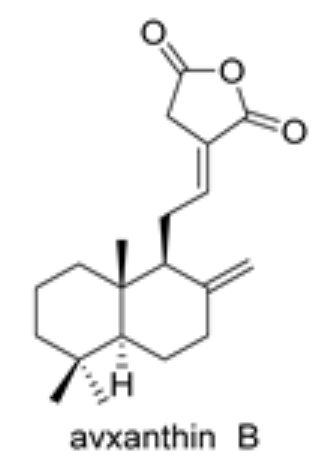
Diterpenic anhydrides were isolated from several plant species. Among them, avxanthin B was isolated from the rhizomes of Amomum villosum. It exhibits significant activity as anti-inflammatory, and an interesting inhibitory activity on α-glucosidase (Molina Inzunza DO et al., Biomolecules 2024, 14, 955).
Devenez membre et participez au développement de la Lipidomique au XXIème siècle.
S'inscrire