
As carelessness at the preliminary stage of extraction may result in the loss of components of interest or in the production of artifacts, the lipidologist must be aware of various precautions that are summarized below.
![]()
SOLVENTS
Neutral lipids or generally storage lipids are extracted with relatively non-polar solvents such as diethyl ether or chloroform but membrane-associated lipids are more polar and require polar solvents such as ethanol or methanol to disrupt hydrogen bondings or electrostatic forces.
To avoid peroxidation of the extracted lipids, during the procedure or later, all solvents should be used peroxide-free. The most convenient is to use “pesticide grade” or HPLC grade” and to buy small quantities at once. Do not stock bottles for a year !! Manufacturers often indicate an expiration date on bottles. To minimize possible deleterious effects due to peroxide formation, pay attention to the expiration dates and keep stocks for no more than 3 months. Furthermore, to avoid contamination of extracted lipids by non-volatile material contained in the large volume of solvent used in the first step of extraction, it is important to use organic solvent containing less than 5 mg dry matter per liter, 1 mg/l is currently proposed.
Diethyl ether, dioxan, isopropyl ether and tetrahydrofuran form peroxides on storage and must be kept in dark bottles or in dark areas. These solvents should be routinely tested to prove that peroxide levels are kept low before use. It must be emphasized that peroxides, even at low levels, may present an explosion hazard after concentration of solvents. Because of its low flammability and its natural origin, ethyl lactate was proposed as a substitute for ethyl acetate in exctracting carotenoids from foods (Ishida BK et al., J Agric Food Chem 2009, 57, 1051).
Alcohols are good solvents for most lipids, methanol and ethanol being the most popular. Ethanol can be contaminated on storage by aldehydes.
Chloroform is a popular solvent, particularly for lipids of intermediate polarity and when mixed with methanol it becomes a general extraction solvent. It is not very stable, forming phosgene and HCl in air, it should be stored in brown bottles. It is sold after addition of preservatives, ethanol, amylene or cyclohexene. Dichloromethane (or methylene dichloride) is a similar extractant but less oxidizable. Chloroform and dichloromethane are currently stabilized by addition of ethanol (up to 1%) or methyl-2-butene-2 (about 20 mg/l).
Among hydrocarbons, hexane is the most popular but is a good solvent only for lipids of low polarity. Its main use is to extract neutral lipids from mixtures of water with alcohols. A mixture of isomers, called “hexanes”, can be used for the same purpose. Hexane can be replaced by petroleum ether which is a mixtures of various hydrocarbons with 5 to 8 carbon atoms. Cyclohexane is sometimes used to store lipid extracts in the cold without danger of evaporation since it freezes at about 6°C. Benzene is no more used since it is now considered as a potent carcinogenic substance, it may be replaced by toluene which, nevertheless, is more difficult to evaporate.
A simple test for peroxides in water-immiscible solvents:
shake 2 ml of solvent with 1 ml of a freshly prepared KI solution (10% in water) and a drop of starch indicator solution, a blue color is rapidly developing if peroxides are present (a faint yellow/brown color after addition of KI indicates low levels of peroxides).
GLASSWARE
The leitmotiv is to avoid any source of contamination by mineral oils, greases, plasticisers from plastics and detergents. Therefore, all operations should be carried out in glass equipment with, if required, ground-glass joints (do not grease !!). Separatory funnels or columns are best equipped with Teflon (PTFE) stopcocks.
All vials or tubes should be closed if necessary (for incubation, agitation, centrifugation) with screw caps including a Teflon-covered liner (Teflon inside the tube !!). Never use cork, rubber, polyethylene or Para film.
Filtration of HPLC solvents should be made with an all-glass vacuum filtration apparatus equipped with filter membranes made of nylon or Teflon.
Except Teflon, all plastics must be avoided because they leach contaminants into the solution which can be detected as unknown spots on thin-layer plates or extra-peaks in GLC chromatograms. Polypropylene tubes can be used if GLC is not necessary for the analysis.
It should be emphasized that all the glassware must be cleaned using classical basic detergents in washing machine or sonicator bath but the washed vials or tubes should be first tested for the absence of contaminants with the current techniques.
REMOVAL OF CONTAMINANTS
In the presence of some water, small molecules are dissolved in organic solvents. Among these contaminants there are pigments, lipophilic amino acids, polypeptides and some hydrophobic proteins.
The removal of contaminants can be done by various ways:
– A simple way to eliminate “proteolipids” is to evaporate the lipid extract under vacuum, add a small amount of methanol which is an excellent denaturation agent and evaporate again. Redissolve the dried lipid extract with a small volume of chloroform/methanol (2/1) and transfer into another clean tube.
– Use the washing procedure of Folch’s method and repeat the process if necessary with the same volume of the reconstituted upper phase. Finally, a chloroform phase can be washed
efficiently with water by vortexing and centrifugation.
– Use a liquid/liquid partitioning column. The Sephadex packing (G 25 superfine) is swollen (20 g/250 ml of solvent) in a mixture of chloroform/methanol/water (60/30/4.5) and washed three times (30 min each time). A small amount of suspension is poured in a small glass column (1 cm diameter, 2.5 cm height for filtration of up to 10 mg lipids). The lipid sample is dissolved in the same solvent mixture and 2.5 ml are applied to the column followed by 5 ml of the same mixture, 2.5 ml of chloroform / methanol (2/1) and finally by 2.5 ml of chloroform / methanol / water (48/35/10). The total purified lipid extract (12.5 ml) is evaporated under a stream of nitrogen and redissolved in the required amount of solvent. This procedure is commonly used for gangliosides studies where colored contaminants frequently hide lipid spots on HPTLC (Dreyfus et al Anal Biochem 1997, 249, 67).
Alternatively, all lipids except gangliosides are separated from various impurities (bile salts, amino acids sugars) on a Sephadex G-25 column previously equilibrated with chloroform/methanol (19/1, v/v) saturated with water. A glass column with a Teflon stopcock and a fiberglass plug is used. The Sephadex beads are equilibrated in 1/1 methanol/water and added to a height of 10 to 20 cm. Five cm of washed sea sand is then added on the top. Lipids dissolved in chloroform/methanol (19/1, v/v) are applied on the column and eluted with 50 ml of the same solvent. The column is regenerated with 1/1 methanol/water and then with 19/1 chloroform/methanol saturated with water and thus can be used indefinitely.
Chlorophyll removal : High levels of chlorophyll-type compounds are frequently found in lipid extracts from plants. The presence of these pigments may interfere with further lipid fractionation. Thus, a method has been developed to chemically remove chlorophyll without modification of other extracted lipids (Bahmaei M et al., JAOCS 2005, 82, 679).
Crude lipid extracts are mixed with a mechanical device at 200 rpm with a 0.4wt% mixture of phosphoric and sulfuric acids (2/0.75, v/v) for 5 min at 50°C. The precipitate is removed from the oily extract by centrifugation (4000 x g, 5 min) and the oil extract is washed according to the Folch procedure to remove acid traces.
PRACTICAL CONSIDERATIONS
Glassware: Since all solvents may extract also some contaminants from containers or apparatus, only Teflon lined stoppers and clean glassware must be used. During extraction take care of any contact between solvent and washers or greased bearings in your mechanical device.
Antioxidant: When fatty acid analyses are planned, it is advisable to add an antioxidant such as butylated hydroxytoluene (BHT) (about 100 mg per liter).
Lyophilized tissues: They are difficult to extract, thus it is recommended to rehydrate them with distilled water before extraction (about 5 ml per g dry material).
With some tissues: it is recommended to add first the required methanol amount during the homogenization to prevent clogging, this is currently the case with liver, muscle, blood. chloroform is then added before the last mixing and the true extraction step.
Evaporation of solvents: When a large amount of solvent must be evaporated, it should done in a rotary film evaporator, the flask containing the extract being maintained at no more than 50°C with a water bath. The reduced pressure must be generated by a pump running without oil (motorized pump with metallic or Teflon membranes) or conveniently with a water aspirator achieving a vacuum of 10-20 mm Hg. Last traces of water may be removed by adding 1 or 2 ml ethanol and evaporating again.
The first description of that concentration device was made by Craig LC (Craig LC et al., Anal Chem 1950, 22, 1462), the famous inventor of countercurrent distribution. The diagram given in the Craig’s paper (see below) shows clearly the principle of the device found on the market as yet.
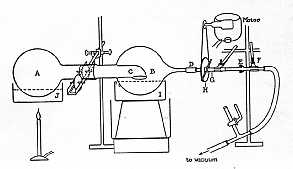
If small samples are extracted, the small solvent volumes (1-5 ml) are easily evaporated under nitrogen blowing and warming the tubes with the help of a water bath or a heating fan. With the later device, an excessive heating must be avoided as some free fatty acids or their esters (C14 and C16) escape from the tubes.
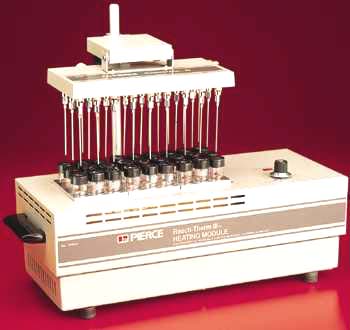
A very valuable and efficient evaporation equipment may be found from Pierce, the Reacti-ThermTM System where reaction vials are heated in a thermostated aluminium block and the Reacti-VapTM Evaporator module enabling a gentle nitrogen blow.
Lipids should not be left for any time in the dry state but should be taken up rapidly in an inert non-alcoholic solvent such as chloroform.
If the weight of extracted lipids are needed, the desiccation time must be prolonged till a constant weight is reached. A convenient procedure is to evaporate a part of the total extract in a small flask and to keep it overnight under vacuum before weighing.
STORAGE OF LIPID EXTRACTS
Lipid extracts should not be stored in the dry state. Oxidative degradation is slower in solutions even in the absence of added antioxidants; This protection depends on the chemical nature of the solvent and the physical conditions of storage.
Air and light should be avoided. The best storage conditions are a super freezer (-70°C) to store for a long time (up to one year) lipid extracts in chloroform-methanol mixtures filling well stoppered glass vials (with Teflon liners), the cap being secured with a wide length of self-sticking tape. Pencil written labels should be protected by an outer layer of polyester tape. For long period of storage, it can be useful to flush vials or tubes with nitrogen before closing to prevent fatty acid oxidation. For the same purpose, a low amount of antioxidant such as BHT (butylated hydroxytoluene or 2,6-di-tert-butyl-4-methoxyphenol) or ethyl gallate (i.e. about 50 µg BHT/ml) may be added in the solvent if the natural antioxidant amount is estimated too low (purified extracts). The antioxidant is used as a concentrated solution in ethanol (10 mg/ml).
After long periods of storage, the cleavage of ester lipids and plasmalogens must be expected with a concomitant rise in free fatty acids, diacylglycerols methyl or ethyl esters. These problems can be minimized by using neutral extracts and 2-propanol instead of methanol or ethanol in the solvent mixture. It must be remembered that best results are obtained with freshly prepared materials.
To prevent labile lipids from oxygen attack it is possible to use a commercially available oxygen absorber (Ageless, Mitsubishi Gas Chem Co) placed inside the sample package. After a short “pretreatment” at 2°C, the labile lipids appear preserved even at room temperature for a long time. This method allows transportation of biomaterial samples to a distant laboratory without utilizing any freezing system (Hirao S et al., J Oleo Sci 2003, 52, 583).
It has been demonstrated that after one year storage of plasma samples at -80°C various lipid parameters (cholesterol, triglycerides) were altered (Devanapalli B et al., Clin Chim Acta 2002, 322, 179). Thus, even at low temperature, intact biological samples must be extracted as soon as possible. It was shown that storing dry tissues in the freezer for more than one month was associated with a decrease in lipids, which is also observed for other storage methods. For qualitative studies of fatty acids (expressed in %), a three-month storage of dry tissue in freezer did not affect the relative composition of species/tissues with a lipid content below 20% of dry weight (Sardenne F et al., Anal Chim Acta 2019, 1051, 82). In contrast, it was shown that after storage at -80°C up to 4 years, the fatty acid compositions of plasma triglycerides and erythrocyte phospholipids were practically unchanged (Hodson L et al., Clin Chim Acta 2002, 321, 63).
Devenez membre et participez au développement de la Lipidomique au XXIème siècle.
S'inscrire