
WAX ESTERS ANALYSIS
In general, as the mixture is too complex, the identification and quantification of wax esters involve a preliminary fractionation of the neutral lipid fraction from the source studied on a column of silicic acid.
The neutral lipid fraction is then separated into classes on another silicic acid column.
The glass column is prepared with 1g (for 10-20 mg lipids) of silicic acid suspended in dichloromethane/hexane (2/3, v/v).
The lipid sample is dried and dissolved in the same solvent (1 ml) and placed on top of the packing. When the solvent is drained, wax esters are eluted by the same solvent (the needed volume is to be determined according to the lipid mass and the wax composition, about 5-10 ml).A larger volume elutes triglycerides.
A pure wax fraction may be isolated by a further TLC in toluene or xylene (Rf: 0.65-0.70) and identified with authentic samples.
Another TLC system may be used to isolate was esters from several chemical derivatives : silica gel plates are developed with a mixture of hexane/diethyl ether (94/6, v/v). Wax had a Rf of about 0.64 (Graille J et al., JAOCS 1986, 63, 111).
The isolation of waxes from vegetal oils is done classically using the International Olive Council method (COI/T.20/Doc. No. 18/Rev. 2, 2003). Briefly, this separation is performed in a glass column filled with hydrated silica gel (15 g, 2% water content) as a solid stationary phase. About 0.5 g of oil mixed with a suitable amount of internal standard solution (0.2% of C32 in n-hexane) and two drops of a 1% solution of the Sudan I dye in n-hexane were loaded into the column with the aid of two 2-mL portions of n-hexane. Under these conditions, the retention of Sudan I dye lies in between that of the waxes and the triacylglycerols fraction. Hence, when the dye reaches the bottom of the chromatographic column, the wax elution is complete. The waxes were eluted with n-hexane/ethyl ether (99:1 v/v). The eluted wax fraction was evaporated to dryness and diluted with n-heptane for chromatographic analysis. By including a double-adsorbent layer of silica gel and silver nitrate-impregnated silica gel in the CC, a more exact determination of wax esters in crude and refined vegetable oils was possible (Carelli AA et al., Eur J Lipid Sci Technol 2012, 114, 1312).
To analyze the wax content of vegetable oils, a simple procedure of purification based on liquid chromatography on a double-adsorbent layer of silica gel and silver nitrate-impregnated silica gel was proposed (Hénon G et al., JAOCS 2001, 78, 401). The hydrocarbons and waxes were then analyzed by gas chromatography
An efficient technique to evaluate the wax ester contant of olive oils using HPLC and GLC has been described (Biederman M et al., Eur Food Res Technol 2008, 228, 65).
Analysis of wax esters
1. It is sometimes advantageous to fractionate wax esters into individual molecular species according to chain length using GLC with capillary column (SE 30 or OV1 type) at high temperature (between 250 and 320°C). The column is standardized for chain length identification with synthetic esters covering the desired range. An example of gas chromatogram of jojoba wax esters is given below.
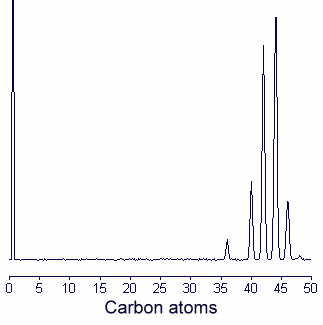
2. Wax esters may be transformed into fatty acid ethyl esters and fatty alcohols by an acid catalyzed ethanolysis which is complete after refluxing 1.5 hr with an anhydrous solution of 1N HCl in ethanol (made in adding acetyl chloride in ethanol).
After recovery in hexane, the mixture is fractionated by TLC or by silicic column chromatography with an elution by dichloromethane/ether (35/1, v/v) which purifies the fatty acid derivatives and an elution by dichloromethane/ether (9/1, v/v) which purifies the residual wax alcohols.
Analysis of the fatty acid ethyl esters are performed as usual by GLC on a polar column.
Analysis of fatty alcohols is performed by GLC on the same column either directly or better as acetate or trimethylsilyl esters.
A reliable and simple method to analyze the composition of jojoba wax by column capillary gas chromatography has been described (Graille J et al., JAOCS 1986, 63, 111). The analysis of the wax ester fraction of vegetal oils by gas chromatography and mass spectrometry was also described in details (Reiter B et al., JAOCS 2001, 78, 881). The use of high temperature gas chromatography and mass spectrometry allowed the detection and identification of a wide range of components which was needed for the characterization of several waxes used in works of art (Regert M et al., J Chromatogr A 2005, 1091, 124). Characterization of waxes used in pictorial artworks was also carried out by chromatographic analysis of fatty acids and hydrocarbons (Peris-Vicente J et al., J Chromatogr A 2006, 1101, 254).
A simple procedure for the determination of waxes present in vegetal oils (from 34 to 46 carbon atoms) has been described (Perez-Camino MC et al., J Chromatogr A 2003, 983, 283). It involves a solid-phase extraction on silica-gel cartridges and a separation by GLC on a dimethyl – diphenylpolysiloxane capillary column. The retention data of higher wax esters within the range C24 to C44 were calculated after gas chromatography, thus allowing the determination of number of carbon atom and the number and position of double bonds in acid and in alcohol moieties of esters (Stransky K et al., J Chromatogr A 2006, 1128, 208).
A more complex equipment is needed when complex mixtures must be analyzed. Thus, the wax ester fraction of edible oils were analyzed by on-line NPLC-GC-MS and by comprehensive two-dimensional GC with flame ionization detection (GC6GC-FID) off-line combined with NPLC-GC (Biederman M et al., Eur J Lipid Sci Technol 2008, 110, 1084). GC6GC-FID enables to group the various classes of wax esters, in particular the phytol esters, geranylgeraniol esters and the straight-chain esters of palmitic acids and the unsaturated C18 acids.
Wax esters of various natural sources have been extensively reviewed:
Nicolaides N et al., Lipids 1970, 5, 299
Kolattukudy PE (Ed.) Chemistry and biochemistry of natural waxes, Elsevier, 1976.
Lee RF et al., Alcohols and waxes, in Marine biogenic lipids, fats and oils. (Ackman RG ed), vol.I, 73-102, CRC Press Inc 1989.
Devenez membre et participez au développement de la Lipidomique au XXIème siècle.
S'inscrire