
STRUCTURAL ANALYSIS
OF TRIACYLGLYCEROLS
Simple regiodistribution analysis of triacylglycerols limited to the distribution of fatty acids to the 2-position and to the 1,3-positions can be made by the analysis of hydrolysis products obtained either by :
or
2- chemical reaction
A comprehensive review of all aspects of the stereospecific analysis of fatty acid distribution was released by Buchgraber M (Eur J Lipid Sci Technol 2004, 106, 621).
![]()
The methodology used the properties of a lipase (pancreatic lipase) which is specific for the primary ester bond of glycerides. The enzyme hydrolyses more rapidly saturated than polyunsaturated fatty acids and short than long chains. However, with triacylglycerols commonly found in animal or vegetal tissues, little fatty acid specificity is evident. Hydrolysis produces fatty acids and mainly 2-monoacylglycerol. Only that form needs to be isolated and transesterified for GLC analysis, since any 1- or 3-monoacylglycerol (migrating in the same TLC spot) are products of acyl migration. Furthermore, released fatty acids may not be similar to the mean composition of the positions 1 (a) and 3 (a‘). This mean composition of each fatty acid is calculated from its proportion in the intact triacylglycerol and in position 2.
To determine quantitatively the fatty acid at the sn-2 position (b), an extensive triglyceride hydrolysis must be processed.
Apparatus
TLC plates (silicagel G) and tank
water bath (40°C)
Vortex
Reagents
– Incubation medium: 1 M Tris buffer pH 8 containing 2.2% CaCl2 (w/v) and 0.05% deoxycholate (w/v) – 10 mg/ml pancreatic lipase (w/v) in water
– 4 M HCl
– diethylether, ethanol, chloroform, chloroform/acetone (96/4, v/v)
– 2.3% boric acid in ethanol, primuline solution, BF3/methanol reagent
Procedure
Evaporate a small volume of solution containing up to 5 mg triacylglycerol in a glass tube.
Add 1.35 ml of the incubation medium and warm at 40°C for 2 min
Add 0.1 ml of pancreatic lipase and vortex for 3 min (do some trials with unknown samples).
Stop the enzymatic reaction by addition of 1 ml ethanol and 1.5 ml 4 M HCl
Wash the aqueous phase two times with 5 ml diethylether, collect the ether phase in a glass tube and wash with 2 ml water.
The ether phase is evaporated and dissolved in 50-100 µl chloroform.
The products are separated by TLC on boric acid impregnated silicagel plates with chloroform/acetone as solvent system.
After spraying primuline, the spot corresponding to 2-monoacylglycerols is scraped off and directly transmethylated with BF3/methanol reagent.

A : origin, B : 1-MG, C : 2-MG, D : fatty acids
E : 1,2-DG, F : 1,3-DG, G : TG
Analyze the methylated fatty acids by GLC (position 2 or b) and, with the composition of an aliquot of the initial triacylglycerol, calculate the position a of each fatty acid by means of the relationship: position a = [(3 x triacylglycerol)-(position b)] / 2.
A critical study of analytical techniques for the determination of the partial positional distribution of fatty acids in triacylglycerols has shown that the method previously described is the most reliable and shows good correlation with fat melting point and hardness (Segura J. et al., Grasas Aceites 2015, 66, e076).
Extensive enzymatic hydrolysis
About 50 mg of fat are hydrolyzed after addition of 40 mg pancreatic lipase in 4 ml of a Tris solution (1M, pH 8.0). Tubes are shaken and 0.4 ml of calcium chloride solution (22%, w/v) and 2 ml of sodium chloride solution (0.1%, w/v) are added. The tubes are incubated at 40°C for 15 min under agitation. After cooling, 2 ml HCl (6M) and 1 ml diethyl ether are added. Tubes are shaken for 1 min and centrifuged. The upper ether layer is collected and evaporated for further analysis of fatty acids or monoglycerides.
A new approach of the asymmetrical stereochemical distribution of fatty acids in triacylglycerols has been described calculating the asymmetric a coefficient from the sn-2 fatty acid, and triacylglycerol composition of the oil (Martinez-Force E et al., Anal Biochem 2004, 334, 175). This coefficient reflects the relative content of fatty acids at the sn-1 and sn-3 positions.
These enzymatic methods have been applied to various vegetable and animal oils with mainly C14-20 fatty acids. Unfortunately, they are not suitable for triacylglycerols containing short chain and polyunsaturated fatty acids, because of lower lipase hydrolysis rates. Thus, an alternative method has been developed using immobilized lipase B from Candida antarctica (Watanabe Y. et al., J Oleo Sci 2015, 64, 1193). The method was applied with success to fish oil, milk fat, and palm oil. Its repeatability and reproducibility have ben collaboratively studied by 12 laboratories.
![]()
As difficulties are due to the occurrence of unavoidable acyl migration, an analytical procedure based on ethyl magnesium bromide deacylation was proposed (Turon F et al. Lipids 2002, 37, 817-821).
This deacylation procedure was shown to lead to representative 2-monoacylglycerols (2-MAG), allowing the composition of the native triacylglycerols in the 2-position to be determined directly. The fatty acid composition in the 1,3-positions can then be estimated from the composition of the 2-MAG and TAG according to the formula 3 x TAG – (2 x 2-MAG).
All details on the experimental procedure, similar to that proposed below, may be found in the original paper (Turon F et al 2002).
This methodology aims at the stereospecific analysis of triacylglycerols. Since no lipase has yet been found that distinguishes between position 1 and 3 of a triacylglycerol molecule, specific chemical and enzymological procedures were devised. Brockerhoff H was the first to propose a complete set of reliable reactions to investigate the stereo specific structure of these compounds (Lipids 1971, 6, 942).
We have used the proposed method, modified from the original procedure of Brokerhoff, for the study of depot fats in rat adipose tissues.
The whole procedure is normally run on two days: the first day diacylglycerols (a mixture of 1,2- and 2,3-sn-isomers) are prepared from the triacylglycerols by a partial hydrolysis with a Grignard reagent. Then, the diacylglycerols are converted synthetically to phospholipids. The second day the phosphatidic acid formed are hydrolyzed by a phospholipase A2. This enzyme reacts only with the 1,2-diacyl-sn-glycerophosphatides and forms a lysophospholipid containing the fatty acids which were present in position sn-1 of the triacylglycerol molecules while the free fatty acids were released from the sn-2 position. After separation by TLC of the products and GLC analysis of the fatty acids, fatty acids in position sn-3 can be calculated.
Apparatus
Glove box flushed with dry air or nitrogen
Vortex, centrifuge, silica gel TLC plates (5721 from Merck) and tanks
Reagents
Diethylether, acetic acid, dry chloroform (kept on molecular sieve), dry pyridine (kept on molecular sieve), methanol, ethanol, 1 M HCl, triethanolamine
Ethyl magnesium bromide in ether, 0.5 M NaHCO3 in water, dry NA2SO4, 2.3% boric acid in ethanol (w/v), POCl3, CaCl2, dimethylaminopyridine (DMAP), 0.4 M EDTA in water, phospholipase A2 from Naja naja
Procedure
First day:
– Triacylglycerols (about 10 mg) are dissolved in 2.8 ml diethyl ether, add 0.2 ml ethyl Mg Br with the help of a 1 ml plastic syringe in a glove box, vortex for 1 min and add 40 µl acetic acid, vortex again 30 s, add 4 ml diethyl ether and 1 ml water, vortex 2 min.
– Centrifuge 5 min and, after removal of the lower phase, add 1 ml NaHCO3 solution, vortex, centrifuge and remove the lower phase.
– Add 1 ml water, vortex and remove the lower phase, add some mg dry Na2SO4 powder, vortex and centrifuge
– Transfer the ether phase in an other tube, evaporate and dissolve in 100 µl chloroform
– Separate diacylglycerols immediatly by TLC on boric acid impregnated plates developed in chloroform/acetone. Localize the 1,2-DAG after primuline spray and detection under UV light and scrape the spots into glass tubes and eluate 2 times the powder with 6 ml diethyl ether which are then washed with 2 ml water. Ether is evaporated and lipids are dissolved in 100 µl chloroform.
– The next step is the synthesis of phosphatidic acid and must be run without delay:
After evaporation of the chloroform, cool the tube on ice, add 950 µl dry chloroform, 950 µl dry pyridine, 100 µl POCl3 and about 1 mg DMAP. Vortex 10 s, keep on ice for 10 min and warm at room temperature for 50 min.
– Add 4 ml chloroform, 2 ml 0.5 M NaHCO3 and 0.4 ml 0.5 M EDTA, vortex 2 h at room temperature
– Centrifuge, collect the lower phase, evaporate and dissolve in 100 µl of chloroform/methanol (2/1, v/v).
Second day:
– Purify PA by TLC on boric acid impregnated plate developed in chloroform/ethanol/water/triethylamine (30/35/8/35, v/v)
– The PA spots are eluted with 2 times 4 ml chloroform/methanol/water (5/5/1, v/v), then add 4 ml water to the extract, vortex, centrifuge and evaporate the lower phase. Dissolve the lipid extract with 100 µl chloroform/methanol (2/1, v/v)
– Evaporate the tubes and add 0.5 ml tris buffer containing 4 mM CaCl2. After a 30 s sonication, add 5 U of Naja naja venom (in Tris buffer/glycerol, 1/1, v/v), 2 ml diethyl ether and vortex 2 h at 20°C.
– Evaporate the ether phase, add 300 µl 1N HCl, 4 ml chloroform/methanol (2/1, v/v) and 1 drp of triethanolamine. Vortex and centrifuge. Remove the upper phase. Add 2 ml methanol/water (1/1, v/v) and vortex.
– The lower phase is evaporated and dissolved by 100 µl chloroform/methanol (2/1, v/v)
– Separate PA, LPA and fatty acids by TLC on boric acid impregnated plates as previously described.
Collect the spots corresponding to LPA and free fatty acids (see chromatogram below) and methylate with BF3. The fatty acid composition of LPA corresponds to position sn-1 and that of the free fatty acids to position sn-2. The fatty acid composition of position sn-3 is not determined directly but can be calculated from the analysis of the original triacylglycerol and those of positions sn-1 and 2. Thus, for each fatty acid, calculate position sn-3= 2 x (triacylglycerol) – (position sn-1) – (position sn-2).
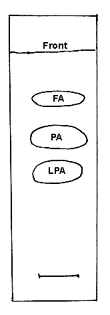
TLC separation of the products of PA hydrolysis by the phospholipase A2 from Naja venom on silica gel plates, solvent system: chloroform/ethanol/water/triethylamine (30/35/8/35, v/v)
Other procedures:
A simple and efficient method for regiospecific analysis of triacylglycerols using only gas chromatography has been proposed (Angers P et al., JAOCS 1999, 76, 481). This method is based on the partial deacylation by a Grignard reagent followed by derivatization of the reaction products with n-butyl chloride and direct analysis of the dibutyrate derivatives of monoglycerides by gas chromatography.
A chromatographic analysis of the structure of natural triglycerides via that of the diacylglycerols derived by Grignard degradation has been proposed as a convenient procedure. Naphthylethylurethane derivatives of the 1,2- and 2,3-diacylglycerols are resolved using normal phase chromatography and may be also identified by mass spectrometry (Agren JJ et al., Lipids 2002, 37, 613).
The lipase from Rhizopus oryzae has been used for the sn-2 position analysis of triacylglycerols containing medium and short chain fatty acids (Perignon M et al., JAOCS 2012, 89, 89-96).
DISPERSIVE LIQUID-LIQUID MICROEXTRACTION
Lire la suiteDevenez membre et participez au développement de la Lipidomique au XXIème siècle.
S'inscrire