
TLC SEPARATION OF ACYLGLYCEROLS
Acylglycerols are readily obtained by column chromatography on silicic acid and in a second step are separated from the majority of the other neutral lipids by TLC.
For routine analysis of lipid samples, we favor the use of silica gel G plates without fluorescent indicator (Merck 5721). After a first migration in chloroform/methanol (1/1, v/v) in a specialized tank followed by a rapid drying, lipids are applied as a chloroform solution as bands (at 1.5 cm from the bottom) and plates are developed in the solvent mixture: hexane/diethyl ether/acetic acid (70/30/1, v/v).
After about one hour, the plates are dried and the lipid spots identified under a UV lamp after spraying a primuline solution (5 mg in 100 ml of acetone/water, 80/20, v/v).
Acylglycerol spots are scraped in glass tubes and, if necessary for further studies, eluted by 2 washes of 2 ml diethyl hexane/ether (1/1, v/v).
All separated neutral lipids may be quantified directly on the thin layer after spraying a solution of rhodamine 6G (0.3 mg per liter) in 95% ethanol. The plates are then scanned with a fluorescent scanner (exc.: 532 nm, em.: 555 nm). The mass of each neutral lipid band is determined by comparing band intensities of unknown samples to dilution curves of standards. A good correlation of the applied lipid mass and scanned intensity was observed for at least the range 20-1000 mg of lipid (Kishimoto K et al., Biochem Biophys Res Comm 2001, 281, 657). In that work, a double-development method with a 30 min drying period between the two developments improved the separation of several simple lipids (acylglycerols, fatty acids, free cholesterol and cholesterol esters).
It should be observed that isomerisation of diacylglycerols occurs during the migration (because of the acid solvent) and the two bands (1,3- and 1,2-DAG) should be combined for the quantification of this class. The presence of cholesterol in the diacylglycerol fraction does not disturb the quantification of the later by fatty acid or glycerol determination, likewise, the presence of traces of primuline does not interfere with further analyses.
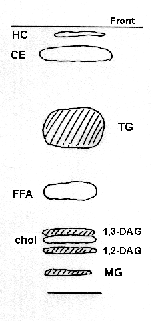
HC: hydrocarbons, CE: cholesterol esters, TG: triacylglycerols,
FFA: free fatty acids, 1,3-DAG: 1,3-diacylglycerols, 1,2-DAG: 1,2-diacylglycerols,
Chol: cholesterol, MG: monoacylglycerols
The migration may vary somewhat in absolute value but the relative order of mobilities is unchanged.
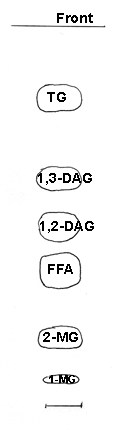
In some special cases, as for structural studies of acylglycerols, it becomes necessary to purify the lipids by chromatographic procedures in which the isomerisation (migration of an acyl group in mono- and diacylglycerols from one carbon of the glycerol to another) is the smallest possible. The simplest method is to separate the lipids by TLC on silica gel plates impregnated with boric acid. A cyclic boric acid derivative fixes the hydroxyl groups, and the relative stability of the complexes determines the separation possible. Di- and monoacylglycerols isomerise easily in acidic solution (and on silica gel), even in the cold, thus, they should be used as soon as possible for derivatization or further chromatography.
Procedure:
Prepare impregnated plates (Merck 5721) by flushing slowly with 2.3% boric acid in ethanol. After draining the liquid, the plates are dried 10 min at 100°C.
After application of the lipid samples, plates are developed in chloroform/acetone (96/4, v/v).
After a short drying time, the lipid spots are detected by primuline spray as before and eluted from the silica gel by two washes with 3 ml diethyl ether. The ether phase is then washed rapidly with 2 ml water to remove traces of boric acid and evaporated. Components are dissolved in the minimum amount of chloroform.
As an example, we give below the aspect of a separation of a vegetal lipids after lipase hydrolysis.
Devenez membre et participez au développement de la Lipidomique au XXIème siècle.
S'inscrire