
FREE STEROLS
ANALYSIS
Cholesterol is the main sterol present in animal tissues but other sterols may be present in biological extracts as those prepared from vegetal.
In animal tissues, the lipidologist is most frequently faced with the determination of cholesterol in complex lipid matrices.
![]()
The choice of a strategy
A. If determinations of other components must be made on the same extract (phospholipids, triacylglycerols..) or if only some little amount of material is available, it is most convenient to separate cholesterol (and sterols) by TLC before its quantification.
After chromatography (TLC), sterols (free and esterified) can be detected and positively identified by means of various spray reagents, the following being a popular and sensitive one.
Procedure: the developed TLC plate is sprayed with a solution freshly prepared of 50 mg ferric chloride (FeCl3) in a mixture of 90 ml water, 5 ml acetic acid and 5 ml sulfuric acid. After heating at 100°C for 3-5 min, the sterol spots are indicated by a red-violet color.
When the cholesterol is detected on one part of the TLC (standard lane), the sample spots are scraped and eluted two times with 2 ml dichloromethane. The solvent is evaporated and the residue is dissolved in a know little amount of the same solvent before the determination of cholesterol by any of the proposed methods.
B. If sterols are the only compounds to be determined in the lipid sample, or if the amount of lipids allows several samplings, it is recommended to do a saponification in alkaline conditions before quantification of sterols. Note that this step converts sterol esters into free sterols. To estimate the free and esterified sterols, see as for cholesterol esters.
Apparatus:
small glass tubes, vortex, heated bath (60°C)
Reagents:
3% KOH in methanol
hexane
Procedure:
An aliquot of plasma (50-100 µl) or lipid extract is saponified by adding 1 ml of KOH solution and heating 15 min at 60°C.
After cooling, add 1 ml water and extract cholesterol by mixing two times with 2 ml hexane. The hexane phase is evaporated and the unsaponifiable fraction is dissolved in 1 ml of dichloromethane.
After these separation and purification steps the lipidologist must choose its quantification approach :
1. In lipid extracts from animals, cholesterol is generally the only sterol to be determined.
If the amount of cholesterol present in a convenient aliquot is estimated to be in the range 1-10 µg, a colorimetric method can be run.
2. If the cholesterol amount is too low to be estimated by colorimetry (range 0.01-1 µg in the aliquot) or if other sterols are present (food or plant extracts) or if all sterol compounds must be quantified, two techniques can be used:
![]()
For the determination of cholesterol in biological solutions or lipid extracts, a simple and sensitive colorimetric method is presented below. As other chemical methods, it has no specificity for one sterol or another, but is very useful for the determination of cholesterol in µg amounts in cellular or plasma lipid extracts. Reliable results are obtained after removing the other lipids (fatty acids) by saponification of the extract before cholesterol determination.
Apparatus:
Small glass tubes, vortex, spectrophotometer
Reagents:
Dichloromethane – o-phthalaldehyde (OPA) – acetic acid – sulfuric acid – cholesterol.
Procedure:
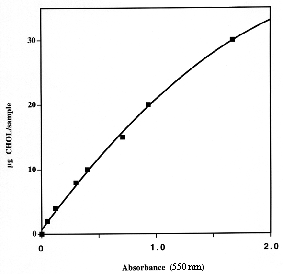
One aliquot of the dichloromethane solution (100-200 µl) is evaporated, then, add 1 ml of a fresh solution of 50 mg OPA in 100 ml pure acetic acid, vortex and after 10 min (in vortexing) add 0.5 ml concentrated sulfuric acid. After 15 min, the absorbance of the unknown and a series of standards and one blank are determined at 550 nm.
Comments:
1 to 5 µg of cholesterol can be accurately determined, we use this method for up to 30 µg cholesterol despite a non-linear dose/absorbance relationship. A linear equation is used from 0 to 10 µg and a polynomial equation from 10 to 30 µg.
|
OTHER ANALYTICAL METHODS |
– Gas chromatographic determination of major sterols in edible oils and fats using solid-phase extraction in sample preparation
Toivo J et al., Chromatographia 1998, 48, 745
– Quantification of cholesterol in foods using non-aqueous capillary electrophoresis
Xu X et al., J chromatogr B 2002, 768, 369
Lipids of food samples are extracted and saponified. Cholesterol is extracted with hexane. The lipid residue is dissolved in the running buffer (100 mM Na acetate in methanol / 100 mM acetic acid in methanol, 19/1). The electrophoresis is carried out at 23.5 kV, 25°C with a fused-silica capillary (50 mm ID x 47 cm) and a Beckman P/ACE 5510 system, the detection is at 210 nm.
The cholesterol retention time is 15 min and the detection limit is 5 mg/ml.
– Analysis of free and esterified sterols in vegetal oils
Verleyen T et al., JAOCS 2002, 79, 117
– A simplified method for the quantification of total cholesterol in lipids using gas chromatography
Hwang BS et al., J Food Comp Anal 2003, 16, 169
100 mg of lipid are saponified, followed by methylation with boron trifluoride. The mixture is extracted with ether, added with an internal standard (5a-cholestane) and analyzed by gas chromatography.
– Solid-phase extraction-thin layer chromatography-gas chromatography method for the detection of hazelnut oil in olive oils by determination of esterified sterols
Cercaci L et al., J Chromatogr A 2003, 985, 211
The oil was subjected to SPE, cold saponification and purification on sillica TLC. The sterol band was analyzed by direct GLC. The sterol fraction provides precise information about the origin of olive oil and a possible admixtures with hazel oil.
An important review of published chromatographic methods for the analysis of plant sterols may be found in the paper by Abidi SL (J Chromatogr A 2001, 935, 173) and that by Volin P (J Chromatogr A 2001, 935, 125).
– Capillary electrochromatography of sterols and related steryl esters derived from vegetable oils
Abidi SL, J Chromatography A 2004, 1059, 199
– Quantitative determination of cholesterol, sitosterol, and sitostanol in cultured Caco-2 cells by liquid chromatography-atmospheric pressure chemical ionization mass spectrometry.
Palmgren JJ et al., J Chromatogr B 2005, 821, 144-152
– Identification of biologically active triterpenes and sterols present in hexane extracts from Miconia species using high-resolution gas chromatography.
Crevelin EJ et al., Biomed Chromatogr 2006, 20, 827-830
– Rapid and quantitative determination of total sterols of plant and animal origin in liver samples by gas chromatography.
Brufau G et al., Chromatographia 2006, 64, 559-563
– A simple and sensitive enzymatic method for cholesterol quantification in macrophages and foam cells.
Robinet P et al., J Lipid Res 2010, 51, 3364-9
– Determination of total sterols in brown algae by Fourier transform infrared spectroscopy.
Bopuzidi N et al., Anal Chim Acta 2008, 616, 185-9
– A simplified method for cholesterol determination in meat and meat products.
Dinh TTN et al., J Food Comp Anal 2008, 21, 306-314
– A simplified method for HPLC-MS analysis of sterols in vegetable oils.
Carretero AS et al., Eur J Lipid Sci Technol 2008, 110, 1142-9
– Application of comprehensive two-dimensional gas chromatography to sterols analysis.
Mitrevski B et al., J Chromatogr A 2008, 1214, 134-142
– Determination of cholesterol in food samples using dispersive liquid-liquid microextraction followed by HPLC-UV.
Daneshfar A et al., J Chromatogr B 2009, 877, 456-460
– Rapid determination of cholesterol in emulsified confectioneries by ultra-performance liquid chromatography.
Ahn JH et al., Eur J Lipid Sci Technol 2012, 114, 1304-11
DISPERSIVE LIQUID-LIQUID MICROEXTRACTION
Lire la suiteDevenez membre et participez au développement de la Lipidomique au XXIème siècle.
S'inscrire