
Preparation and purification of ceramides
1- Isolation of free ceramides
If present in sufficient amount, ceramides may be directly purified by a two step procedure including a separation by column chromatography combined with TLC.
The separation on a silica gel column, as previously described, enables the elution with acetone/methanol of a fraction containing all the glycolipids and the ceramides. Another procedure was previously described where ceramides are purified as a fraction of neutral lipids. After evaporation of the solvent, the lipid extract may be chromatographed on a silica gel plate to isolate a ceramide spot.
Separation by TLC of free ceramides
Dissolve the lipid extract in a small volume of chloroform/methanol 2/1 (v/v).
Separate on normal silica gel plates with:
– a first migration with chloroform/methanol/acetic acid, 190/9/1 (v/v) as mobile phase
– a second migration with diethyl ether/acetic acid, 100/1 (v/v) as mobile phase
Spots are localized with the help of commercial standards after primuline spray and eluted by washing the scraped silica gel two times with chloroform/methanol (2/1).
Ceramides either found in free form in cellular extracts or sampled after preparation from more complex lipids are easily studied after derivatization to enable their further quantification or analysis.
Immediately after lipid extraction from tissues or cells, ceramides are directly derivatized with naproxen as for diacylglycerols to give fluorescent (and UV absorbing) compounds. After TLC, the spot corresponding to ceramides is eluted and the extract is thus available for quantification or molecular species analysis.
2 – Preparation of ceramides from sphingomyelin
A– Ceramides are prepared from sphingomyelin by hydrolysis using phospholipase C from C. welchii as for the production of DAG from other phospholipids and derivatized with naproxen as previously described.
B– It must be recalled that ceramide-containing sphingomyelin may be prepared directly from a crude lipid extract by mild saponification if other compounds are of no interest.
Mild saponification procedure:
The dried lipids are warmed 30 min at 45°C with an adapted volume of methanol/20 N NaOH in water (19/1, v/v). After cooling, add 2 volumes chloroform and 1 volume water. Vortex and centrifuge at low speed. The lower phase is collected and evaporated for the study of ceramide-containing lipids. If the sample contains relatively moderate amounts of glycolipids, the phospholipase hydrolysis can be run directly on the saponified extract. For more precise works, it is convenient to isolate first sphingomyelin from the other sphingolipids by column chromatography.
A less complex saponification procedure can be used when fatty acids do not interfere with subsequent analyses. The methylamine hydrolysis, processed according to the first step of the procedure previously described for the deacylation of polyphosphoinositides (hydrolysis followed by evaporation of the reaction medium), prevents any liquid/liquid partition and yields intact sphingolipids.
A modified procedure may be also used: dissolve and warm the dry lipid extract with 0.5 ml of 25% monomethylamine in ethanol at 50°C for 30 min. The alkali-resistant extract is dried under nitrogen and suspended in an appropriate volume of chloroform/methanol (2/1, v/v).
One of the most efficient purification method for all sphingolipids has been proposed for shotgun lipidomics analyses (Jiang X et al., Anal Biochem 2007, 371, 135). Briefly, Briefly, a small portion of each individual lipid extract was evaporated under a stream of nitrogen. A small volume of ice-cooled LiOMe solution (1M, 50 ml) in methanol was added to the test tube at 0°C. The reaction mixture was vortexed for 15 s, placed in an ice bath for 1 h, and quenched with 2 ml of 0.4% acetic acid solution. The aqueous phase was washed with hexane (2 ml, 3 times). The lipids in the aqueous phase were extracted by the modified Bligh and Dyer. The combined chloroform phase was dried under a stream of nitrogen. The residue was reextracted against a 10 mM aqueous LiCl solution and each extract was dissolved in 100 ml of CHCl3/MeOH (1/1, v/v).
3 – Preparation of ceramides from glycosphingolipids
Ceramides are less easily prepared from glycosphingolipids than from sphingomyelin. Compounds containing dihydroxy bases (sphingosine, dihydrosphingosine, sphingadienine) can be converted to ceramides by the procedure proposed by Carter et al (J Lipid Res 1961, 2, 227). This process consists in an opening of the glycosidic ring with periodate and the resulting product is chemically reduced before its hydrolysis under mild acidic conditions. For trihydroxy base-containing sphingolipids present in vegetals, only enzymatic hydrolysis (b-glucosidase) can be performed but without completion of the reaction (Gatt, Methods in Enzymol 1969, 14, 152).
Chemical generation of ceramides from glycosphingolipids
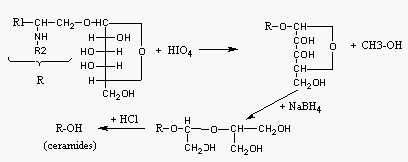
Glycosphingolipid hydrolysis (Method of Carter)
Reagents
Chloroform, 95% ethanol
5M NaOH, 0.1M NaOH, 2M HCl
0.2M HIO4 in water (freshly prepared), NaBH4 (10 mg/ml 0.1M NaOH)
100-200 mesh Florisil
Procedure
The quantities are given for the preparation of ceramides from 1g crude glycosphingolipids but they can be easily adapted for any amount of lipids.
Step 1- Lipids (1 g) are dissolved in 7 ml chloroform and 28 ml ethanol are added.
After dissolution, add under agitation 15 ml of HIO4 solution and keep the mixture 24 h in the dark. Then, the lipids are extracted by adding 15 ml chloroform and 5 ml water under agitation. After centrifugation and a washing of the lower phase with 5 ml water, the chloroform phase is evaporated.
Step 2- The dry lipid extract is dissolved by adding successively 28 ml ethanol, 10 ml water, 0.1 ml 5M NaOH and 5 ml NaBH4 solution. Keep 3 h in the dark. Acidify the solution by adding slowly 2M HCl (about 1.2 ml) with the help of phenolphthalein indicator. Add 25 ml water and collect the lower phase which is evaporated.
Step 3- The dry extract is dissolved by adding 3 ml chloroform, 6.5 ml methanol, 0.25 ml water and 0.25 ml 4M HCl. 24h later, the mixture is evaporated and redissolved in chloroform (warm if necessary).
Step 4- Ceramides are purified by column chromatography on Florisil.
We used a column (diameter: 2 cm) containing 20 g 100-200 mesh Florisil, previously activated one night at 110°C. Pour the powder as a suspension in chloroform.
Wash the column with 100 ml chloroform and elute ceramides with 300 ml chloroform/methanol (19/1, v/v).
Devenez membre et participez au développement de la Lipidomique au XXIème siècle.
S'inscrire